Recently Posted
-

AItris for Buchanan Optometrists
 Mark Braddon
3 min.
Mark Braddon
3 min.Disclaimer: In the USA, Altris Image Management System (Altris IMS) has USA FDA 510(k) Class II clearance; AI/ML models and components are intended to use for research purposes only, not for clinical diagnosis purposes.
Buchanan Optometrists and Audiologists is no ordinary eye-care center.
The Association of Optometrists (AOP) estimates 17,500 registered optometrists working across roughly 6,000 practices in the UK. The UK Optician Awards recognise the best in the UK Optical industry. To even make the top 5 is our equivalent of an Oscar nomination! They are the only practice in the UK to consistently make the top 5 since 2008. Buchanan Optometrists describe themselves as innovators who “continually push boundaries.”
Their list of awards speaks for itself:
- 2012 – National Optician Award for Premium Lens Practice of the Year
- 2013 – Luxury Eyewear Retailer of the Year and Premium Lens Practice of the Year
- 2013 – Winner at the UK Optician Awards
- 2015–2016 – Best UK Independent Practice
- 2017–2018 – Optometrist of the Year, with Alisdair Buchanan named the top optometrist in the UK
- 2023–2024 – Best Independent Optician and Best Technology Practice
And this list is not finished, as Alisdair Buchanan, the Owner and the Director of the center, is investing in their growth continuously.

With a track record like this, it’s no surprise that Buchanan Optometrists was among the first to adopt AI for Decision Support in OCT. AI is rapidly becoming a vital part of modern eye care, and leading centers are already embracing it.
Mark Braddon, Altris AI VP of Clinical Sales, sat down with Alisdair Buchanan, the owner and director of the practice, to talk about his experience with AI and what it means for the future of optometry.
Mark Braddon: You’ve been working with OCT for years. What changed in your practice after bringing in Altris AI Decision Support for OCT?
Alisdair Buchanan, Owner: As someone already confident in interpreting scans, I didn’t need help understanding OCT—but Altris provides something even more valuable: a kind of second opinion. It supports my clinical decisions and offers an added layer of reassurance, particularly in borderline or complex cases. That’s not just helpful—it’s powerful.
I didn’t think our OCT assessments could improve much—until we started using Altris AI. It’s not just an upgrade; it’s become an indispensable part of delivering modern, high-quality eye care. Altris AI has significantly enhanced the way we interpret OCT scans. What used to require prolonged focus and cross-referencing now takes moments, without sacrificing accuracy or depth. The system analyses images with incredible precision, highlighting subtle pathological changes that are often time-consuming to detect, especially during a busy clinic day.

Mark Braddon: What was the first real benefit you noticed after bringing Altris AI into your day-to-day routine?
Alisdair Buchanan, Owner: One of the most immediate benefits has been in patient communication. The platform generates clear, colour-coded visuals that make explaining findings effortless. Instead of trying to talk patients through grainy greyscale images, we can now show them precisely what we’re seeing. It’s improved understanding, reduced anxiety, and increased trust in the care we’re providing.
Mark Braddon: Was it easy to fit AI Decision Support into your OCT workflow? How easy did you find integrating Altris AI?
Alisdair Buchanan, Owner: Integration was seamless—no faff, no friction. It fits naturally into our existing workflow, with scans uploaded and analysed within seconds. It’s helped us work more efficiently, without compromising the thoroughness our patients expect.
In short, Altris AI has sharpened our clinical edge and strengthened the service we offer. It doesn’t replace experience—it enhances it. And that, for me, is the real value.
Mark Braddon: In your experience, where has AI been the most helpful in clinical work?
Alisdair Buchanan, Owner: The main area where it shines is in picking up early macular changes, particularly dry AMD. Things like drusen or subtle changes in the outer retinal layers, which could easily be missed at a glance, are brought to the surface immediately.

It’s also been handy with diabetic patients. Just having that extra layer of input to flag microstructural changes helps us stay ahead of progression.
We’ve also started using it with glaucoma suspects. While our Heidelberg Spectralis remains our go-to for structural monitoring, having the RNFL analysis from Altris adds a checkpoint. I’d never base a referral purely on it, but it’s nice to have a second opinion—even if it’s an AI one.
Mark Braddon: Has AI Decision Support changed how you handle borderline or difficult-to-call cases?
Alisdair Buchanan, Owner: I’d say it’s given us more confidence, particularly in the grey areas—those borderline cases where you’re not quite sure if it’s time to refer or just monitor a bit more closely. With AMD, for example, it has helped us catch early signs of progression and refer patients before things become urgent.
And for glaucoma, again, it’s not replacing anything we do—it’s just another tool we can lean on. Sometimes it confirms what we already thought, and other times it nudges us to look again more carefully.
Mark Braddon: How has using AI impacted your conversations with patients during consultations?
Alisdair Buchanan, Owner: One of the unexpected benefits has been how much it helps with patient conversations. We show the scans on-screen during the consultation, and the colour overlays make things much easier to explain, especially with older patients. They can see what we’re talking about, which makes the whole thing feel more real and less abstract.
They often say, “Ah, now I understand,” or “So that’s what you’re looking at.” It’s not about dazzling them with tech—it just helps make the discussion more transparent and more reassuring.

Mark Braddon: Some professionals worry that AI might replace human judgment. How do you see its role in clinical decision-making?
Alisdair Buchanan, Owner: I don’t see Altris —or any AI—as a threat to what we do. It’s not here to replace us. We still make the decisions, take responsibility, and guide our patients. But it does help.
For me, it’s like having a quiet assistant in the background. It doesn’t get everything right, and I certainly wouldn’t act on it blindly—but it prompts me to pause, double-check, and sometimes spot something I might have missed otherwise. That can only be a good thing.
In short, Altris has sharpened our clinical edge and strengthened the service we offer. It doesn’t replace experience—it enhances it. And that, for me, is the real value.
-

AI for Decision Support with OCT: “Altris Gave Me More Certainty in My Clinical Decisions”
 Maria Martynova
2 minutes
Maria Martynova
2 minutesDisclaimer: In the USA, Altris Image Management System (Altris IMS) has USA FDA 510(k) Class II clearance; AI/ML models and components are intended to use for research purposes only, not for clinical diagnosis purposes.
AI for Decision Support with OCT: An Interview with Clara Pereira, Optometrist from Franco Oculista
About Franco Oculista Optometry in Portugal.
Franco Oculista is the optometry center with a 70-year-old history: its roots date back to the mid-1950s in Luanda, where it was founded by Gonçalo Viana Franco. Having left behind a career in pharmacy, Gonçalo pursued his entrepreneurial vision by opening an optician’s bearing his name in the heart of the Angolan capital. Driven by a thirst for knowledge and a deep sense of dedication, he turned his dream into reality. With a commitment to professionalism and a forward-thinking approach, he integrated the most innovative technologies available at the time. This blend of passion, expertise, and innovation established Franco Oculista as a benchmark for quality and excellence in the field. In 1970s, the family returned to Portugal and opened the new FRANCO OCULISTA space on Avenida da Liberdade.
How do Franco Oculista describe their mission?
“Through individualized and segmented service, we seek to respond to the needs of each client. We combine our knowledge with the most sophisticated technical equipment and choose quality and reliable brands. We prioritize the evolution of our services and, for this reason, we work daily to satisfy and retain our customers with the utmost professionalism.”

Clara Pereira is one of the optometrists at Franco Oculista and has been an optometrist for nearly two decades. Based in a private clinic in Portugal, she brings years of experience and calm confidence to her consultations. We talked with her to learn how her clinical practice has evolved, particularly since integrating OCT and, more recently, Altris AI – AI for Decision Support with OCT.
Altris AI: Clara, can you tell us a bit about your daily work?
Clara: “Of course. I’ve been working as an optometrist for 19 years now. My practice is quite comprehensive—I assess refractive status, binocular vision, check the anterior segment with a slit lamp, measure intraocular pressure, and always examine the fundus.
Clara: “In Portugal, we face limitations. We’re not allowed to prescribe medication or perform cycloplegia, so imaging becomes crucial. I rely heavily on fundus photography and OCT to guide referrals and detect early pathology.”

Altris AI: How central is OCT diagnostics to your workflow?
Clara: “OCT is substantial. I perform an OCT exam on nearly every patient, on average, eight OCT exams per day. It’s an essential part of how I gather information. With just one scan, I can learn so much about eye health.”Altris AI: What kind of conditions do you encounter most frequently?
Clara: “The most common diagnosis is epiretinal membrane—fibrosis. But I also manage patients with macular degeneration and other retinal pathologies. Having the right tools is key.”Altris AI: And what OCT features do you use the most?
Clara: “I regularly use the Retina, Glaucoma, and Macula maps. But if I had to choose one, the Retina Map gives me the most complete picture. It’s become my go-to.”Altris AI: You’ve recently started using Altris AI. What has that experience been like?
Clara: “At first, I didn’t know much about it. But when Optometron introduced Altris AI to me—a company I trust—I didn’t hesitate. And I’m glad I didn’t. From the beginning, it felt like a natural extension of my clinical reasoning.Clara: “Altris AI gives me an extra layer of certainty. It helps me extract more from the OCT images. I usually interpret the scan myself first, and then I run it through the platform. That way, I validate my thinking while also learning something new.”
Altris AI: Have any standout cases where Altris AI made a difference?
Clara: “Yes. I’ve had a few. One was a case of advanced macular degeneration, in which the AI visualization really helped me explain the condition to the patient. Another was using anterior segment maps for fitting scleral lenses—Altris was incredibly useful there, too. I do a lot of specialty lens fittings, so that was a big advantage.”
Altris AI: Would you recommend Altris AI to your colleagues?
Clara: “I would recommend Altris AI to my colleagues. For me, it’s about more than just the diagnosis. It’s about feeling confident that I’m seeing everything clearly and giving my patients the best care possible. Altris AI helps me do exactly that.”
Why This Matters: Altris AI in Real Practice
Clara’s story reflects the real value of AI in optometry—not as a replacement for clinical judgment, but as a powerful companion. With every OCT scan, she strengthens her expertise, improves diagnostic accuracy, and gives her patients the reassurance they deserve.
Whether identifying early signs of fibrosis, supporting complex scleral lens fittings, or acting as a second opinion, Altris AI seamlessly fits into the modern optometrist’s workflow, making every scan more meaningful.
AI for Decision Support with OCT: Transforming Retinal Diagnostics
Artificial Intelligence (AI) is revolutionizing the field of ophthalmology, particularly through its integration with Optical Coherence Tomography (OCT). OCT is a non-invasive imaging technique that captures high-resolution cross-sectional images of the retina, enabling early detection and monitoring of various ocular conditions. However, interpreting these scans requires time, expertise, and consistency—factors that AI-based decision support systems are uniquely positioned to enhance.
Altris AI (AI for OCT decision support platform) analyzes thousands of data points across B-scans, automatically detecting retinal pathologies, quantifying biomarkers, and identifying patterns that may be subtle or overlooked by the human eye. By providing objective, standardized assessments, Altris AI reduces diagnostic variability and improves clinical accuracy, especially in busy or high-volume practices.
For optometrists and ophthalmologists, AI acts as a second opinion, flagging early signs of diseases such as age-related macular degeneration (AMD), diabetic retinopathy, and glaucoma. It streamlines workflows by highlighting areas of concern, prioritizing cases that require urgent attention, and offering visual explanations that are easy to communicate to patients.
Moreover, Altris AI enableS longitudinal tracking of pathology progression. By comparing OCT scans over time ( even from various OCT devices), clinicians can monitor subtle changes in drusen volume, retinal thickness, supporting timely clinical decisions and tailored treatment strategies. The integration of AI into OCT interpretation not only enhances diagnostic confidence but also supports evidence-based care, early intervention, and improved patient outcomes. As AI continues to evolve, it will play a vital role in advancing precision medicine in ophthalmology, empowering eye care professionals with tools that are fast, reliable, and scalable.

In essence, AI for OCT decision support is not replacing clinical expertise; it is augmenting it, elevating the standard of care through speed, accuracy, and actionable insights.
-

Future of Ophthalmology: 2025 Top Trends
 Maria Znamenska
13.03.202512 min read
Maria Znamenska
13.03.202512 min readFuture of Ophthalmology: 2025 Top Trends
In a recent survey conducted by our team, we asked eye care specialists to identify the most transformative trends in ophthalmology by 2025. The results highlighted several key areas, with artificial intelligence (AI) emerging as the clear frontrunner, cited by 78% of respondents.

However, the survey also underscored the significant impact of optogenetics, novel AMD/GA therapies, and the continuing evolution of anti-VEGF treatments. This article will explore the practical implications of these advancements, providing an overview of how they are poised to reshape diagnosis, treatment, research, and, ultimately, patient outcomes in ophthalmology.
In this article, we will also discuss Oculomics, a very promising field that is gaining momentum.
Top AI Technology for Detecting Eye-related Health Risks 2025
Building upon the survey’s findings, we begin with the most prevalent trend: top AI technology for detecting eye-related health risks in 2025

AI in Clinical Eye Care Practice
With the increasing prevalence of conditions like diabetic retinopathy and age-related macular degeneration, there is a growing need for efficient and accurate screening tools. And AI is already valuable for eye-care screening: algorithms can analyze retinal images and OCT scans to identify signs of these diseases, enabling early detection and timely intervention.

AI-powered screening tools can also help identify rare inherited retinal dystrophies, such as Vitelliform dystrophy and Macular telangiectasia type 2. These conditions can be challenging to diagnose, but AI algorithms can analyze retinal images to detect subtle signs that human observers may miss.
AI also starts to play a crucial role in glaucoma management. Early detection of glaucoma demands exceptional precision, as the early signs are often subtle and difficult to detect. Another significant challenge in glaucoma screening is the high rate of false positive referrals, which can lead to unnecessary appointments in secondary care and cause anxiety for patients, yet delayed or missed detection of glaucoma results in irreversible vision loss for millions of people worldwide. So, automated AI-powered glaucoma analysis can offer transformative potential to improve patient outcomes.
This OD module evaluates optic disc parameters using OCT, providing personalized assessments by accounting for individual disc sizes and angle of rim absence. Such a tailored approach eliminates reliance on normative databases, making evaluations more accurate and patient-specific.
Furthermore, it enables cross-evaluation across different OCT systems, allowing practitioners to analyze macula and optic disc pathology, even when data originates from multiple OCT devices. Key parameters evaluated by Altris AI’s Optic Disc Analysis include disc area, cup area, cup volume, minimal and maximum cup depth, cup/disc area ratio, rim absence angle, and disc damage likelihood scale (DDLS).

AI for Clinical Trials and Research
AI is revolutionizing clinical trials and research in ophthalmology. One such key application of AI is biomarker discovery and analysis. Algorithms can analyze large datasets of medical images, such as OCT scans, to identify and quantify biomarkers for various eye diseases. These biomarkers can be used to assess disease progression, monitor treatment response, and predict clinical outcomes.
AI is also being used to improve the efficiency and effectiveness of clinical trials. By automating the process of identifying eligible patients for clinical trials, AI can help researchers recruit participants more quickly and ensure that trials include appropriate patient populations, accelerating the development of new treatments.

Algorithms can analyze real-world data (RWD) collected from electronic health records and other sources to generate real-world evidence (RWE). RWE provides valuable insights into disease progression, treatment patterns, and long-term outcomes in everyday clinical settings, complementing the findings of traditional randomized controlled trials.
Oculomics
Integrating digitized big data and computational power in multimodal imaging techniques has presented a unique opportunity to characterize macroscopic and microscopic ophthalmic features associated with health and disease, a field known as oculomics. To date, early detection of dementia and prognostic evaluation of cerebrovascular disease based on oculomics has been realized. Exploiting ophthalmic imaging in this way provides insights beyond traditional ocular observations.

For example, the NeurEYE research program, led by the University of Edinburgh, is using AI to analyze millions of anonymized eye scans to identify biomarkers for Alzheimer’s disease and other neurodegenerative conditions. This research can potentially revolutionize early detection and intervention for these devastating diseases.
Another effort spearheaded by researchers from Penn Medicine, Penn Engineering is exploring the use of AI to analyze retinal images for biomarkers indicative of cardiovascular risk. AI systems are being trained on fundus photography to detect crucial indicators, such as elevated HbA1c levels, a hallmark of high blood sugar, and a significant risk factor for both diabetes and cardiovascular diseases.

AI analysis of retinal characteristics, such as retinal thinning, vascularity reduction, corneal nerve fiber damage, and eye movement, has shown promise in predicting Neurodegenerative diseases. Specifically, decreases in retinal vascular fractal dimension and vascular density have been identified as potential biomarkers for early cognitive impairment, while reductions in the retinal arteriole-to-venular ratio correlate with later stages.
Moving from AI, we now turn to another significant trend identified in our survey:
Optogenetics
Optogenetics represents a significant leap forward in ophthalmic therapeutics, offering a potential solution for vision restoration in patients with advanced retinal degenerative diseases, where traditional gene therapy often falls short. While gene replacement therapies are constrained by the need for viable target cells and the complexity of multi-gene disorders like retinitis pigmentosa (RP), optogenetics offers a broader approach.

This technique aims to circumvent the loss of photoreceptors by introducing light-sensitive proteins, known as opsins, into the surviving inner retinal cells and optic nerve, restoring visual function through light modulation. This method is particularly advantageous as it is agnostic to the specific genetic cause of retinal degeneration.
By delivering opsin genes to retinal neurons, the technology enables the precise manipulation of cellular activity, essentially transforming these cells into new light-sensing units. This approach can bypass the damaged photoreceptor layer, transmitting visual signals directly to the brain.
Several companies are pioneering advancements in this field. RhyGaze, for example, has secured substantial funding to accelerate the development of its lead clinical candidate, a novel gene therapy designed for optogenetic vision restoration. Their efforts encompass preclinical testing, including pharmacology and toxicology studies, an observational study to define clinical endpoints, and a first-in-human trial to assess safety and efficacy. The success of RhyGaze’s research could pave the way for widespread clinical applications, significantly impacting the treatment of blindness globally.

Nanoscope Therapeutics is also making significant strides with its MCO-010 therapy. This investigational treatment, administered through a single intravitreal injection, delivers the Multi-Characteristic Opsin (MCO) gene, enabling remaining retinal cells to function as new light-sensing cells. Unlike earlier optogenetic therapies that required bulky external devices, MCO-010 eliminates the need for high-tech goggles, simplifying the treatment process and enhancing patient convenience. The ability to restore light sensitivity without external devices represents a major advancement, potentially broadening the applicability of optogenetics to a wider patient population.

Another critical area of innovation highlighted in our survey is the advancement of treatments for AMD and GA.
New AMD/GA Treatment
Age-related macular degeneration (AMD) and geographic atrophy (GA) represent a significant challenge in ophthalmology, demanding innovative therapeutic strategies beyond the established anti-VEGF paradigm.

Gene Correction
Gene editing is emerging as a powerful tool in the fight against AMD and GA, potentially correcting the underlying genetic errors that contribute to these diseases. Essentially, it allows us to make precise changes to a patient’s DNA.
Traditional gene editing techniques often rely on creating ‘double-strand breaks’ (DSBs) in the DNA at specific target sites, which are like precise cuts in the DNA strand. These cuts are made using specialized enzymes, like CRISPR-Cas9, which act as molecular scissors. While effective, these methods can sometimes introduce unwanted changes at the cut site, such as small insertions or deletions.
After a DSB is made, the cell’s natural repair mechanisms kick in. There are two main pathways:
- Non-Homologous End Joining (NHEJ): This is the cell’s quick-fix method. It essentially glues the broken ends back together. However, this process can sometimes introduce errors, leading to small insertions or deletions that can disrupt the gene’s function.
- Homology-Directed Repair (HDR): This is a more precise repair method. It uses a ‘donor’ DNA template to guide the repair process, ensuring accuracy. However, HDR is more complex and less efficient, especially in non-dividing cells.
To overcome these limitations of traditional gene editing, researchers have developed more precise techniques:
- Base Editing: This technique allows scientists to change a single ‘letter’ in the DNA code without creating DSBs.
- Prime Editing: This advanced technique builds upon CRISPR-Cas9, allowing for a wider range of precise DNA changes. It can correct most disease-causing mutations with enhanced safety and accuracy.
- CASTs (CRISPR-associated transposases): This method enables larger DNA modifications without creating DSBs, offering a safer approach to genetic correction.
Why does this matter for AMD and GA? These advancements in gene editing are crucial for addressing the genetic roots of these pathologies. We can potentially develop more effective and targeted therapies by precisely correcting the faulty genes that contribute to these diseases. The technologies are still being researched, but they hold great promise for the future of ophthalmology.
Cell Reprogramming
Cell reprogramming offers a novel approach to regenerative medicine, with the potential to replace damaged retinal cells. This technique involves changing a cell’s fate, either in vitro or in vivo. In vitro reprogramming involves extracting cells, reprogramming them in a laboratory, and then transplanting them back into the patient. In vivo reprogramming, which directly reprograms cells within the body, holds particular promise for retinal diseases. This approach has succeeded in preclinical studies, demonstrating the potential to restore vision in conditions like congenital blindness.

Vectors and Delivery Methods
The success of gene therapy relies on efficiently delivering therapeutic genes to target retinal cells. Vectors are essentially delivery vehicles, designed to carry therapeutic genes into cells. These vectors can be broadly classified into two categories: viral and non-viral. Vectors, both viral and non-viral, are crucial for this process.
Viral vectors are modified viruses that have been engineered to remove their harmful components and replace them with therapeutic genes. They are highly efficient at delivering genes into cells, as they have evolved to do just that. Adeno-associated viruses (AAVs) are the most commonly used viral vectors in ocular gene therapy due to their safety profile and cell-specificity. The diversity of AAV serotypes allows for tailored gene delivery to specific retinal cell types.
Non-viral vectors, on the other hand, are synthetic systems that don’t rely on viruses. They can be made from lipids, polymers, or even DNA itself. While they may be less efficient than viral vectors, they offer safety and ease of production advantages.
Advances in vector design, whether viral or non-viral, are focused on enhancing gene expression, cell-specificity, and carrying capacity.
Now, let’s examine the ongoing evolution of anti-VEGF treatments, a cornerstone of modern retinal care.
New Anti-VEGF drugs
The landscape of ophthalmology has undergone a dramatic transformation since the early 1970s when Judah Folkman first proposed the concept of tumor angiogenesis. His idea sparked research that ultimately led to the identification of vascular endothelial growth factor (VEGF) in 1989 and the development of anti-VEGF therapies, revolutionizing the treatment of neovascular eye diseases, dramatically improving outcomes for patients with wet AMD, diabetic retinopathy, and retinal vein occlusions.
Population-based studies have shown a substantial reduction (up to 47%) in blindness due to wet AMD since the introduction of anti-VEGF therapies. However, significant gaps remain despite this progress, especially regarding treatment durability. Anti-VEGF drugs require frequent intravitreal injections, which can be difficult for patients due to time commitments, financial costs, and potential discomfort. Although newer agents have extended treatment intervals, patient adherence and undertreatment challenges persist in real-world settings. Innovative approaches are being investigated to address these unmet needs to increase drug durability and reduce the treatment burden.
Tyrosine Kinase Inhibitors
One approach to increasing treatment durability is using tyrosine kinase inhibitors (TKIs). TKIs are small-molecule drugs that act as pan-VEGF blockers by binding directly to VEGF receptor sites inside cells, offering a different action mechanism than traditional anti-VEGF drugs that target circulating VEGF proteins.
Currently, TKIs are being investigated as maintenance therapy, primarily in conjunction with sustained-release delivery systems. Two promising TKIs for retinal diseases are axitinib and vorolanib. In a bioresorbable hydrogel implant, Axitinib is being studied for neovascular AMD and diabetic retinopathy. Vorolanib, in a sustained-release delivery system, is also being investigated for neovascular AMD. These TKIs offer the potential for less frequent dosing, reducing the treatment burden for patients.
Port Delivery System
The Port Delivery System (PDS) is a surgically implanted, refillable device that provides continuous ranibizumab delivery for up to 6 months. While it’s FDA-approved for neovascular AMD, it’s also being investigated for other retinal diseases, such as diabetic macular edema and diabetic retinopathy.
Although the PDS faced a voluntary recall due to issues with septum dislodgment, it has returned to the market with modifications. The PDS offers the potential for significantly reduced treatment frequency for a subset of patients. However, challenges remain, including the need for meticulous surgical implantation and the risk of endophthalmitis.
Nanotechnology
Nanotechnology offers promising solutions to overcome limitations of current ocular drug delivery. The unique structure of the eye, with its various barriers, poses challenges for drug delivery. Topical administration often fails to achieve therapeutic concentrations, while frequent intravitreal injections carry risks. Nanotechnology can improve drug solubility, permeation, and bioavailability through nanoparticles, potentially extending drug residence time and reducing the need for frequent injections. Several nanoparticle systems, lipid and polymeric, are being studied for ocular drug delivery, offering hope for more effective and less invasive treatments.
Summing up
The advancements discussed in this article, encompassing AI, optogenetics, novel AMD/GA therapies, and refined anti-VEGF treatments, collectively signal a transformative era for ophthalmology. As highlighted by the survey results, AI probably encompasses most of the changes by redefining diagnostic and clinical workflows through its capacity for image analysis, biomarker identification, and personalized patient management.
Optogenetics offers a distinct pathway to vision restoration, bypassing limitations of traditional gene therapy. The progress in AMD/GA treatments, particularly gene editing and cell reprogramming, presents opportunities for targeted interventions. Finally, the evolution of anti-VEGF therapies, with innovations in drug delivery and sustained-release mechanisms, addresses persistent challenges in managing neovascular diseases.
These developments, driven by technological innovation and clinical research, promise to enhance patient outcomes and reshape the future of ophthalmic care.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

ML Applied to 3D Optic Disc Analysis for Glaucoma Risk Assessment Across Different OCT Scan Protocols Without a Normative Database
 Angelina Hramatik
14.02.202520 min read
Angelina Hramatik
14.02.202520 min readMachine Learning Applied to 3D Optic Disc Analysis for Glaucoma Risk Assessment Across Different OCT Scan Protocols Without a Normative Database
1. Introduction
Glaucoma is one of the leading causes of irreversible blindness worldwide, affecting millions of people annually. The disease is often asymptomatic in its early stages, making timely diagnosis particularly challenging. Early detection of glaucomatous changes is crucial for preventing vision loss and improving long-term patient outcomes.
One well-established method for assessing glaucoma is the Disc Damage Likelihood Scale (DDLS), which evaluates structural changes in the optic nerve head (ONH) based on the extent of neuroretinal rim loss. This method categorizes glaucomatous damage severity by analyzing the relationship between the optic cup and neural rim, while also accounting for optic disc size without relying on a normative database. 1, 2, 3, 4.
While DDLS is recognized for its reliability and utility in clinical practice, it is not a standalone diagnostic tool. Rather, it is one of several methods used to identify signs of glaucoma, and its implementation is often limited to specific imaging modalities or scan protocols, such as 3D optic disc-only scans or fundus images.
In this article, we introduce an enhanced approach to DDLS analysis that overcomes these limitations. We want to present a solution, which is capable of performing DDLS analysis on any OCT scan protocol that captures the optic nerve, including 3D optic disc scans (which provide the most detailed view of the nerve), as well as OCT horizontal and vertical 3D wide scans. By leveraging advanced machine learning models, we achieve unprecedented flexibility and accuracy, ensuring reliable analysis across different scanning protocols and OCT systems.
Unlike traditional systems restricted to specific devices or data formats, our solution processes scans from multiple OCT systems. Moreover, it excels in challenging scenarios, providing clinicians with a robust and versatile tool for analyzing potential signs of glaucoma.
A Brief Theoretical Overview
Optical coherence tomography (OCT) scans vary in the anatomical regions they capture. One specific type is the optic disc OCT scan (Figure 2), which provides high-resolution imaging of the optic disc and the surrounding optic nerve head (ONH) structures. This scan type is commonly used in glaucoma assessment, as it allows for the evaluation of the optic nerve’s structure, including the neuroretinal rim, optic cup, and surrounding peripapillary retinal nerve fiber layer (RNFL) — key areas affected in glaucomatous damage.

Figure 1. Photograph of the retina of the human eye, with overlay diagrams showing the positions and sizes of the macula, fovea, and optic disc (Reference).

Figure 2. 6 mm OCT b-scan of the optic nerve head (ONH) region.
In contrast, macular OCT scans (Figure 3) focus on the central retina, providing detailed visualization of structures such as the foveal center, retinal layers, and macular biomarkers (such as drusen, hypertransmission, fluids etc). Since the macula is anatomically distinct from the optic nerve head, standard macular scans do not capture the ONH comprehensively.

Figure 3. 6 mm OCT b-scan of the macular region, showing the foveal pit and retinal layers.
A more comprehensive scanning approach is 12 mm wide scan OCT (Figure 4), which captures both the macular region and optic nerve head in a single scan. This broader field of view allows for the simultaneous assessment of central retinal structures and optic nerve-related changes, making it valuable for detecting and monitoring conditions that affect both regions, such as glaucoma and other neurodegenerative or vascular retinal diseases.

Figure 4. 12 mm wide scan OCT b-scan, which captures both the macular region and part of the optic nerve head.
2. Results
2.1. Experiment Setup
Brief Method Overview
To evaluate the effectiveness of DDLS analysis in assessing glaucoma severity, we designed an experiment comparing results obtained from processing 3D Optic Disc OCT scans and 3D Wide scan OCT scans with the corresponding reports generated by the OCT system. Our method follows four key steps:
- Detecting optic nerve landmarks like Bruch’s Membrane Opening (BMO) points (Eye Keypoints Retrieval / OCT Keypoint Detector Model);
- Segmenting the inner limiting membrane (ILM) (Retina Layers Segmentation Model);
- Reconstructing the neuroretinal rim geometry;
- Applying the Disc Damage Likelihood Scale (DDLS) for classification.
The dataset below was used to validate the algorithm.
Dataset Used for Validating the Entire Algorithm
For validation, we compared our algorithm’s DDLS measurements with the DDLS values generated by the built-in algorithms of the Optopol REVO NX 130 OCT system. This provided a baseline for assessing accuracy and consistency.
To validate our approach, we conducted an experiment comparing DDLS metrics derived from:
- 3D Optic Disc OCT scans, which are traditionally used for DDLS analysis.
- 3D Wide scans, which capture both the macular and optic nerve regions, providing a more comprehensive dataset for analysis.
The dataset includes imaging data from 37 patients examined using the Optopol REVO NX 130 OCT system, with each patient undergoing the following protocols on the same day:
- 3D Optic Disc OCT (6mm zone): 168 scans
- 3D Wide scan (horizontal protocol, 12mm): 128 scans
A report was obtained from the 3D Optic Disc OCT scans, containing all parameters calculated by the device.
Since no manual annotations are available for these data, our comparison is conducted directly against the device-generated results.
The distribution of data was as follows:
- Glaucomatous Optic Disc: 21 cases;
- Normal Optic Disc: 16 cases.
2.2. Final Validation Results: DDLS Accuracy and Error Metrics
To evaluate the performance of our DDLS analysis method, we compared its results with the corresponding DDLS values generated by the OCT device’s built-in algorithms. The device reports serve as a reference point for all calculations, meaning the accuracy, MAE/STD values presented below indicate the level of agreement between our method and the device’s measurements.
The parameters compared below are the key indicators for glaucoma stage assessment.
- The rim-to-disc ratio (RDR) represents the thinnest neuroretinal rim width relative to the vertical optic disc diameter. A lower RDR indicates a more advanced stage of rim thinning, as glaucoma leads to progressive narrowing of the neuroretinal rim due to the loss of ganglion cells axons.
- The rim absence angle (RAA) quantifies the extent of neuroretinal rim loss in degrees. It defines the angle where the rim is completely absent, exposing the optic cup. A wider RAA suggests a more severe stage of glaucoma, as it indicates greater rim loss across the disc circumference.
Both RDR and RAA provide complementary perspectives on structural optic nerve damage:
- RDR measures the smallest remaining rim thickness in proportion to the disc.
- RAA evaluates how much of the disc circumference has lost its rim.
By considering both parameters together, a more comprehensive assessment of glaucoma severity can be achieved. Based on RDR and RAA, a DDLS stage is assigned, allowing for standardized classification of glaucoma progression.

Table 1. Validation Results of DDLS Analysis on 3D Optic Disc and 3D Wide Scan OCT Scans
The table presents validation results comparing 3D Optic Disc OCT scan and 3D Wide scan OCT in DDLS analysis, focusing on Mean Absolute Error (MAE) and Standard Deviation (STD) for key parameters, along with overall DDLS staging accuracy. These metrics are calculated for the rim-to-disc ratio and rim absence angle by comparing their respective values from 3D Optic Disc OCT scans and 3D Wide scans against those from the device reports, providing a precise assessment of deviations from the reference values.
Key Observations
1. Our Goal: Consistency with Device Reports, Not Outperformance
The experiment does not aim to surpass the device’s accuracy but rather to demonstrate that our method produces results in alignment with the device-generated DDLS reports.
The device report serves as a reference, helping to interpret the figures we present, but this does not mean the device’s output is always the absolute truth.
2. High DDLS Staging Accuracy for Both Scan Types
3D Optic Disc OCT scan: 97.3% accuracy in determining DDLS glaucoma stage.
3D Wide scan OCT: 94.59% accuracy, demonstrating strong reliability despite a broader scan area and fewer scans capturing the nerve, leading to less available information.
Conclusion:
- Both types of scans allow the production of clinically reliable DDLS results, but as expected, 3D optic disc scans provide slightly better accuracy due to their higher resolution of the optic nerve head (ONH).
- The small accuracy gap and close values for key parameters between the two suggests that 3D wide scan OCT can still be a viable option for glaucoma assessment, despite offering less detailed information about the optic nerve compared to optic disc scans.
3. RD Ratio and Rim Absence Angle: High Precision Within Clinical Margins
RD Ratio (rim-to-disc ratio):
- Step size between DDLS stages: 0.1.
- Mean Absolute Error (3D Optic Disc OCT scan): 0.008 (significantly smaller than step size).
- Mean Absolute Error (3D Wide scan OCT): 0.024 (still relatively small).
Conclusion:
- Both 3D Optic Disc OCT scan and 3D Wide scan analysis provide high precision in RD ratio calculations.
- The small error ensures that stage classification remains reliable, especially in optic disc scans.
Rim Absence Angle:
- Step size between DDLS stages: Minimum 45°.
- Mean Absolute Error (3D Optic Disc OCT scan): 2.2° (very small compared to step size). Mean Absolute Error (3D Wide scan OCT): 4.2° (still well below stage transition threshold).
Conclusion:
- The method’s margin of error is far smaller than the clinical threshold for stage differentiation, confirming high accuracy in rim loss assessment.
- 3D Optic Disc scans again show better precision, reinforcing that they remain the preferred scan type for DDLS.
4. Our Advantage: Ability to Perform DDLS on Both Scan Types
- Unlike traditional DDLS implementations, which work only with 3D Optic Disc scans, our method can perform DDLS analysis on both 3D Wide scan and 3D Optic Disc OCTs.
- However, 3D Optic Disc OCT remains the preferred method for maximum precision, as it provides a higher-resolution view of the optic nerve.
Key Conclusions
- Our method is unique in its ability to process multiple scan types, while still maintaining high accuracy in both cases.
- On 3D Optic Disc scans, we achieve maximum precision, while on 3D Wide scans, we still maintain clinically reliable accuracy.
- Consistency: Across all glaucoma stages, our method produced stable results that closely matched ground truths provided by medical experts.
- Universal Compatibility: The algorithm performed equally well with scans from other manufacturers, demonstrating its versatility and robustness.
2.3. Patient Case Studies: DDLS Analysis in Real-World Scenarios
Accurate assessment of glaucoma severity relies on precise measurements of optic nerve parameters, such as disc area, rim-to-disc ratio, and rim absence angle. In the following examples, we analyzed four patient cases, including both normal optic discs and glaucomatous eyes, using 3D Optic Disc OCT scan, 3D Wide scan OCT, and device-generated reports as a reference standard.
By consolidating individual patient cases into a single comparative table, we can examine the consistency of DDLS analysis across different scan types and highlight key variations that may arise due to differences in scan coverage, segmentation accuracy, and anatomical structure. The following table summarizes the key optic nerve parameters measured for each patient and scan type.

Table 2. Comparative DDLS Evaluation Across Multiple Patient Cases
Key Findings & Interpretation
1. High Consistency Between Our Method and Device Reports
- Across all cases, the DDLS stage remains identical (4 for normal eyes, 7 or 8 for glaucomatous cases) regardless of whether the input scan was 3D Optic Disc OCT or wide scan, and this result corresponds to the device-generated report.
- Key optic nerve parameters such as disc area, cup area, and rim area closely align with the device reference, demonstrating strong algorithm performance.
2. Minor Variations in Cup and Rim Measurements
- Cup and rim area values show slight deviations between 3D Optic Disc OCT scans and 3D Wide scan scans, which is expected due to differences in scan coverage and segmentation sensitivity.
- For example, in Patient 3 (Glaucoma, Stage 8):
- Cup area was 1.86 mm² (3D Optic Disc OCT scan), 1.88 mm² (3D Wide scan), and 1.81 mm² (Device Report).
- Rim area was 0.55 mm² (3D Optic Disc OCT scan), 0.53 mm² (3D Wide scan), and 0.58 mm² (Device Report).
- These small variations do not affect final DDLS staging but highlight how scan type can introduce subtle segmentation differences.
3. Rim Absence Angle Varies Slightly but Remains Within Expected Tolerances
- The rim absence angle shows minor fluctuations across scan types, especially in glaucomatous cases.
- Example: In Patient 3 (Stage 8 Glaucoma), the device reported a rim absence angle of 162°, while our algorithm calculated 155° (3D Optic Disc OCT scan) and 151° (3D Wide scan).
- Since DDLS categories for severe glaucoma are defined in large increments (e.g., 45°+ thresholds), these small differences do not impact staging accuracy.
4. 3D Wide scan OCT Provides Comparable Results to 3D Optic Disc OCT scan
- Despite covering a larger field of view, wide scans produced DDLS staging results consistent with 3D Optic Disc OCT scans and device reports.
- In patients with coexisting macular pathologies, 3D Wide scan OCT may provide additional clinical insights while still maintaining high reliability for glaucoma staging.
Conclusion: Reliable DDLS Analysis Across Different Scan Types
This unified case study analysis confirms that our DDLS analysis algorithm produces highly consistent results across different scan protocols and patient conditions.
- DDLS stage assignment is identical to device reports across all scan types, ensuring high agreement with clinically validated reference values.
- Key optic nerve measurements (disc area, cup area, rim area) are closely aligned across 3D Optic Disc OCT scan, 3D Wide scan, and device reports, reinforcing algorithm accuracy.
- Minor variations in rim absence angle and segmentation metrics do not affect final glaucoma staging, highlighting the algorithm’s robustness.
- 3D Wide scan OCT offers a viable alternative for 3D Optic Disc OCT scans, particularly in cases where both macular and optic nerve regions need simultaneous evaluation.
5. Visual Comparison Shows Strong Similarity to Device Reports
- The disk and cup boundaries detected by our algorithm closely match those in the device-generated reports, maintaining consistent shapes and anatomical alignment across both 3D Optic Disc and 3D Wide scan OCT scans.
- However, wide scan-based segmentations tend to be slightly rougher, as less structural information is available compared to dedicated optic disc scans. This trade-off is expected due to the broader field of view in wide scans.
These findings validate our algorithm’s flexibility, adaptability, and clinical reliability, demonstrating its potential for seamless integration into real-world ophthalmic workflows.
2.4. Why Our Approach Stands Out: Key Advantages Over Traditional DDLS Systems
While the previous patient case studies demonstrated the accuracy and consistency of our DDLS analysis across different optic disc conditions, another critical advantage of our method is its ability to work seamlessly across various scanning protocols. Unlike traditional device-restricted solutions, our approach supports DDLS assessment on both standard 3D Optic Disc OCT scans and 3D Wide scans with different orientations.
The following table illustrates the same patient’s optic nerve head analyzed using three different scanning protocols: 3D Optic Disc OCT scan, 3D Wide scan Horizontal, and 3D Wide scan Vertical. This comparison highlights the method’s adaptability to different scan formats, ensuring reliable DDLS analysis regardless of the scanning protocol used. This example is taken from a Topcon Maestro 2 OCT system, providing an additional reference for processing across different OCT systems.

Table 3. Comparative DDLS Analysis Across Different Scanning Protocols: 3D Optic Disc OCT, 3D Wide scan Horizontal, and 3D Wide scan Vertical.
This capability significantly enhances clinical applicability, allowing our algorithm to process data from various scanning protocols and devices while maintaining high accuracy. The ability to analyze both 3D Optic Disc and 3D Wide scan OCT scans — across different orientations and machine types — ensures comprehensive glaucoma assessment even in cases where scan availability or quality may vary.
Key advantages over traditional DDLS analysis methods
1. Device Independence
- While most existing solutions are restricted to proprietary OCT data formats, our algorithm processes scans from any OCT system, ensuring broad compatibility across devices.
2. Consistent Accuracy Across Different Scan Types
- Our algorithm closely matches device-generated DDLS reports, achieving 97.3% accuracy for 3D Optic Disc OCT scans and 94.59% for 3D Wide scan OCTs.
- Patient cases confirm this consistency, with both normal and glaucomatous eyes correctly classified, even when analyzed with different scan types.
3. Robust Performance in Edge Cases
- Unlike traditional device-based DDLS assessments, which may struggle with low-quality images or atypical anatomical features, our approach maintains high accuracy in challenging clinical scenarios.
- Patient examples with small optic discs and advanced-stage glaucoma demonstrated that our algorithm successfully identified key DDLS indicators even when scan quality or nerve structure was less distinct.
4. Expanded Assessment Through 3D Wide scan OCT
- The ability to perform DDLS analysis on Horizontal and Vertical 3D Wide scans allows for a more comprehensive evaluation by incorporating both macular and optic nerve data.
- In patients with coexisting macular pathologies, wide scans enabled earlier detection of glaucomatous changes that would have been missed if only optic disc scans were used.
3. Detailed Approach Description
To assess glaucoma stage on OCT scans using DDLS analysis, the following steps should be performed:
- Optic Nerve Landmarks Detection – Localization of the optic nerve in the b-scan view of each scan by identifying key anatomical landmarks.
- ILM Detection – Segmentation of the inner limiting membrane (ILM) in the b-scan view of each scan to establish a reference for neuroretinal rim measurement.
- Neuroretinal Rim Reconstruction – Construction of the neuroretinal rim geometry based on detected nerve landmarks and ILM segmentation.
- DDLS Analysis – Application of the Disc Damage Likelihood Scale (DDLS) to assess glaucoma severity based on neuroretinal rim measurements. This includes assigning a DDLS stage according to rim width and optic disc size, with a focus on detecting localized thinning and asymmetry.
3.1. Keypoint Annotation Process / Nerve Detection
The foundation of our approach lies in a high-quality, annotated dataset meticulously labeled by a team of four expert ophthalmologists. The annotation process focused on identifying key anatomical landmarks in both the macular region and the optic disc nerve zones, both of which are critical for detecting glaucomatous changes and performing Disc Damage Likelihood Scale (DDLS) analysis.
These keypoints serve as essential data for evaluating disease progression and training machine learning models. The dataset was carefully selected based on key clinical features, such as the presence or absence of nerve fibers, foveal pits, and other pathological markers, ensuring a comprehensive representation of various conditions and scan types.
The annotated dataset consists of approximately 370 unique OCT examinations with more than 56,000 b-scans, covering a range of physical scanning areas, pathology types, and optic nerve conditions to enhance the model’s robustness. The scans are categorized as follows:
- Optic Disc with no excavation: ~15 examinations;
- Glaucomatous Optic Disc: ~105 examinations;
- Normal Optic Disc: ~105 examinations;
- Wide scans (covering both the macular and optic nerve regions): ~60 examinations;
- Normal Retina Scans: ~40 examinations;
- Pathological Retina Scans: ~45 examinations.
This detailed annotation process ensures high precision and reliability, enabling the algorithm to generalize across diverse cases while maintaining clinical accuracy in real-world scenarios.
3.2. Eye Keypoints Retrieval / OCT Keypoint Detector
Our keypoint detection model represents a logical evolution of the model for exam center detection, designed to efficiently and accurately identify key anatomical landmarks in OCT scans. The architecture integrates elements from UNet 5 and CenterNet 6, incorporating YOLO-inspired 7 techniques for keypoint prediction. Additionally, the backbone has been adapted to a transformer-based model 8, enhancing feature extraction capabilities.
Training Process
The training process follows a multi-stage approach, ensuring robustness, accuracy, and efficiency:
- Stage 1: Detects general keypoints, establishing a foundation for precise landmark localization.
- Stage 2: Groups and refines the identification of specific keypoints, progressively improving the model’s understanding of anatomical structures.
This structured approach enhances the model’s reliability across different scan types while maintaining computational efficiency.
Key Features
Data Preprocessing
- The data is augmented using unsupervised techniques, leveraging libraries such as Albumentations 9 to introduce variations such as rotations, scaling, and noise addition.
- This ensures the model encounters a wider variety of real-world scenarios during training, improving its generalization capability.
Training Process
- The model is trained using supervised learning techniques, optimizing a loss function through backpropagation and gradient descent.
- This approach allows for continuous refinement and adaptation to complex variations in OCT scans.
Parameterization & Tuning
- The model includes millions of adjustable parameters (weights), which are fine-tuned to increase accuracy.
- Key hyperparameters such as learning rate, batch size, and network depth are carefully selected to maximize performance.
- Advanced optimization techniques, including grid search, random search, and Bayesian optimization, are used to find the best hyperparameter configuration.
3.3. Retina Layers Segmentation Model
The Retina Layers Segmentation Model is our production-stage model, actively used within the Altris AI platform. It was incorporated into this experiment without modifications, ensuring that the results reflect real-world performance as seen in our deployed system.
Our Retina Layers Segmentation Model enables precise segmentation of key retinal layers in OCT scans, crucial for detecting structural changes linked to glaucoma and other retinal diseases. The model identifies:
- ILM, RNFL, GCL, IPL, INL, OPL, ONL, ELM, MZ, EZ, OS, RPE, BM
The training dataset consists of 5,000 expert-annotated OCT b-scans, covering a diverse range of patient demographics, including different ages and ethnic backgrounds. The segmentation model is designed to detect and delineate key retinal layers with high accuracy.
Training & Architecture
The model is based on U-Net with a ResNet backbone, optimized for OCT images. Training includes:
- Expert Annotation: Medical specialists labeled layers for ground truth.
- Augmentation: Albumentations-based transformations enhance robustness.
- Supervised Learning: Predicts segmentation masks using backpropagation.
- Hyperparameter Optimization: Grid search, random search, and Bayesian tuning maximize performance.
Model Validation & Performance
- The model was validated using a holdout validation approach, with separate validation and test sets that were not exposed during training.
- Real-world testing was conducted using scans from various clinical settings to ensure robustness.
- Performance was evaluated using the Mean Dice Coefficient across all layers, achieving a score of 0.80, with layer-specific scores ranging from 0.63 to 0.92, confirming high segmentation accuracy.
- Cross-domain testing demonstrated consistent performance across different OCT systems, and stability was confirmed over scans collected across different time periods.
This efficient, accurate, and generalizable model strengthens DDLS analysis and enhances AI-driven retinal diagnostics.
3.4. DDLS Algorithm
The DDLS algorithm evaluates glaucomatous changes by analyzing the geometric relationship between the neural rim and optic cup in the optic nerve head. Key steps include:
- Localization: Identifying boundaries of the optic cup and neuroretinal rim by reconstructing geometry on a b-scan view using disc landmarks and an inner limiting membrane.

Figure 5. B-scan Geometry Visualization.
- Measurement: Calculating the DDLS stage based on the ratio between the rim and disc boundaries.
- Cross-Scan Application: Adapting the analysis for 3D Wide scans (both Horizontal and Vertical protocols) as well as 3D Optic Disc-specific scans.
Our implementation enhances this traditional method by leveraging wide scans, enabling a more comprehensive assessment of glaucomatous changes.
3.5. Evaluation
To ensure the reliability and effectiveness of our DDLS algorithm, we conducted a rigorous evaluation process, adhering to best practices in data usage, ethics, and performance validation.
Data Integrity
- Measures were implemented to prevent data leakage, ensuring that scans from the same patient did not appear in both training and testing sets.
Ethical Considerations
- The analysis strictly relies on OCT-related data (e.g., scan zone size, laterality, pixel spacing) without incorporating any personal patient information.
Performance Metrics
- Keypoint detection accuracy was evaluated using Mean Squared Error (MSE), comparing model-predicted keypoints with expert annotations.
- Additional metrics included correctness of scan center-related landmarks and accuracy in the optic nerve region, ensuring precision in clinical applications.
The evaluation results confirmed the algorithm’s robustness, demonstrating significant performance gains, particularly in edge cases, where traditional methods often struggle.
Discussion
Our DDLS analysis method represents a significant advancement in glaucoma detection. Key benefits include:
- Universal Compatibility: The ability to process data from various devices ensures broad applicability.
- Enhanced Accuracy: By incorporating data from both macular and optic nerve regions, our approach captures more subtle glaucomatous changes.
- Edge Case Performance: Advanced machine learning techniques enable accurate analysis even in challenging scenarios.
Compared to traditional methods, our system provides a more flexible, reliable, and comprehensive solution for early glaucoma detection.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
Conclusion
By integrating 3D Wide scans and state-of-the-art machine learning models, we have enhanced DDLS analysis for glaucoma detection, ensuring high accuracy, broad compatibility, and robustness across diverse clinical scenarios.
Unlike traditional solutions, our algorithm:
- Works across multiple OCT devices, eliminating the constraints of proprietary data formats.
- It closely matches device-generated DDLS reports, achieving 97.3% accuracy for 3D Optic Disc OCT scans and 94.59% for 3D Wide scans.
- Performs reliably in edge cases, such as small optic discs and advanced-stage glaucoma, where traditional methods may struggle.
- Supports both Horizontal and Vertical 3D Wide scans, enabling more comprehensive assessments that incorporate both macular and optic nerve data.
- Enhances early glaucoma detection, particularly in patients with coexisting macular pathologies, where wide scans provide additional clinical insights.
By delivering consistently accurate DDLS staging, regardless of scan type or manufacturer, our system establishes a new benchmark for universal glaucoma assessment. This technology has the potential to significantly improve early detection and management, ultimately preserving vision and enhancing patient outcomes.
References
- Spaeth, G. L. (2005). The Disc Damage Likelihood Scale. Glaucoma Today. https://glaucomatoday.com/articles/2005-jan-feb/0105_18.html
- Cheng, K. K. W., & Tatham, A. J. (2021). Spotlight on the Disc-Damage Likelihood Scale (DDLS). Clinical Ophthalmology, 15, 4059–4071. https://pmc.ncbi.nlm.nih.gov/articles/PMC8504474/
- Zangalli, C., Gupta, S. R., & Spaeth, G. L. (2011). The disc as the basis of treatment for glaucoma. Saudi Journal of Ophthalmology, 25(4), 381-387. https://www.sciencedirect.com/science/article/pii/S1319453411000993
- Review of Optometry Staff. (2023, January 23). Optic disc staging systems effective in grading advanced glaucoma. Review of Optometry. https://www.reviewofoptometry.com/article/optic-disc-staging-systems-effective-in-grading-advanced-glaucoma
- Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. [Preprint]. Posted May 18, 2015. https://arxiv.org/abs/1505.04597
- Duan K, Bai S, Xie L, et al. CenterNet: Keypoint Triplets for Object Detection. [Preprint]. Posted April 17, 2019. https://arxiv.org/abs/1904.08189
- Redmon J, Divvala S, Girshick R, Farhadi A. You Only Look Once: Unified, Real-Time Object Detection. [Preprint]. Posted June 8, 2015. https://arxiv.org/abs/1506.02640
- Dosovitskiy A, Beyer L, Kolesnikov A, et al. An Image is Worth 16×16 Words: Transformers for Image Recognition at Scale. [Preprint]. Posted October 22, 2020. https://arxiv.org/abs/2010.11929
- Buslaev A, Iglovikov V, Khvedchenya E, et al. Albumentations: Fast and Flexible Image Augmentations. [Preprint]. Posted September 18, 2018. https://arxiv.org/abs/1809.06839
-

Altris Introduces Next-Generation Fluids and GA Quantification Features
 Maria Znamenska, MD, PhD Ophthalmology
1 min. read
Maria Znamenska, MD, PhD Ophthalmology
1 min. readAltris Introduces Next-Generation Fluids and GA Quantification Features
Altris AI, a pioneering force in artificial intelligence for OCT scan analysis, has unveiled additional quantification features for Fluids and Geographic Atrophy (GA) tracking on its web platform. Altris AI currently detects over 70 retina pathologies and biomarkers. However, we have decided to enhance its capabilities by adding additional Fluids and GA quantification and tracking functionalities, recognizing that eye care specialists frequently work with these conditions.
These advancements empower eye care professionals (ECPs) with cutting-edge tools for diagnosing and managing retinal diseases. By integrating AI-driven quantitative tracking and progression monitoring, Altris AI enables specialists to deliver more personalized and effective treatments, ultimately enhancing patient outcomes.
Fluids Quantification and Progression Tracking
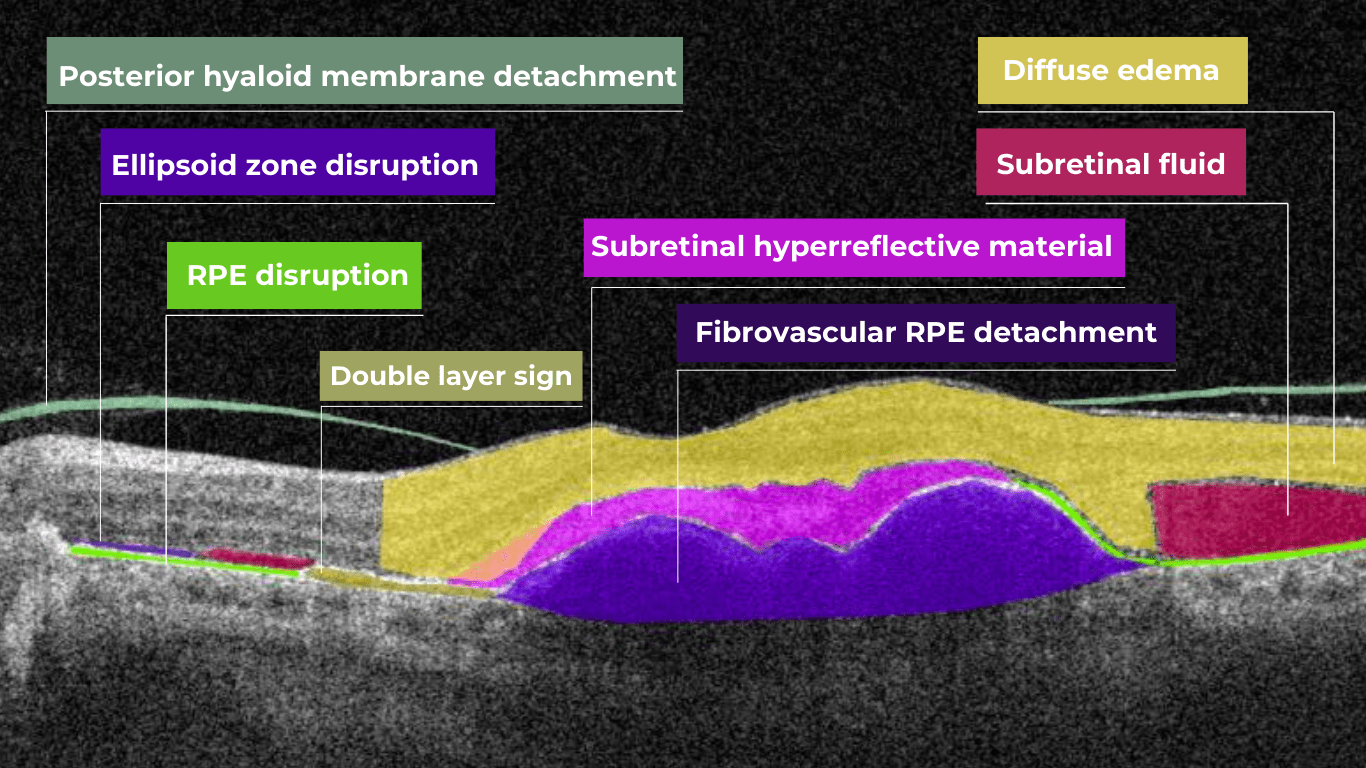
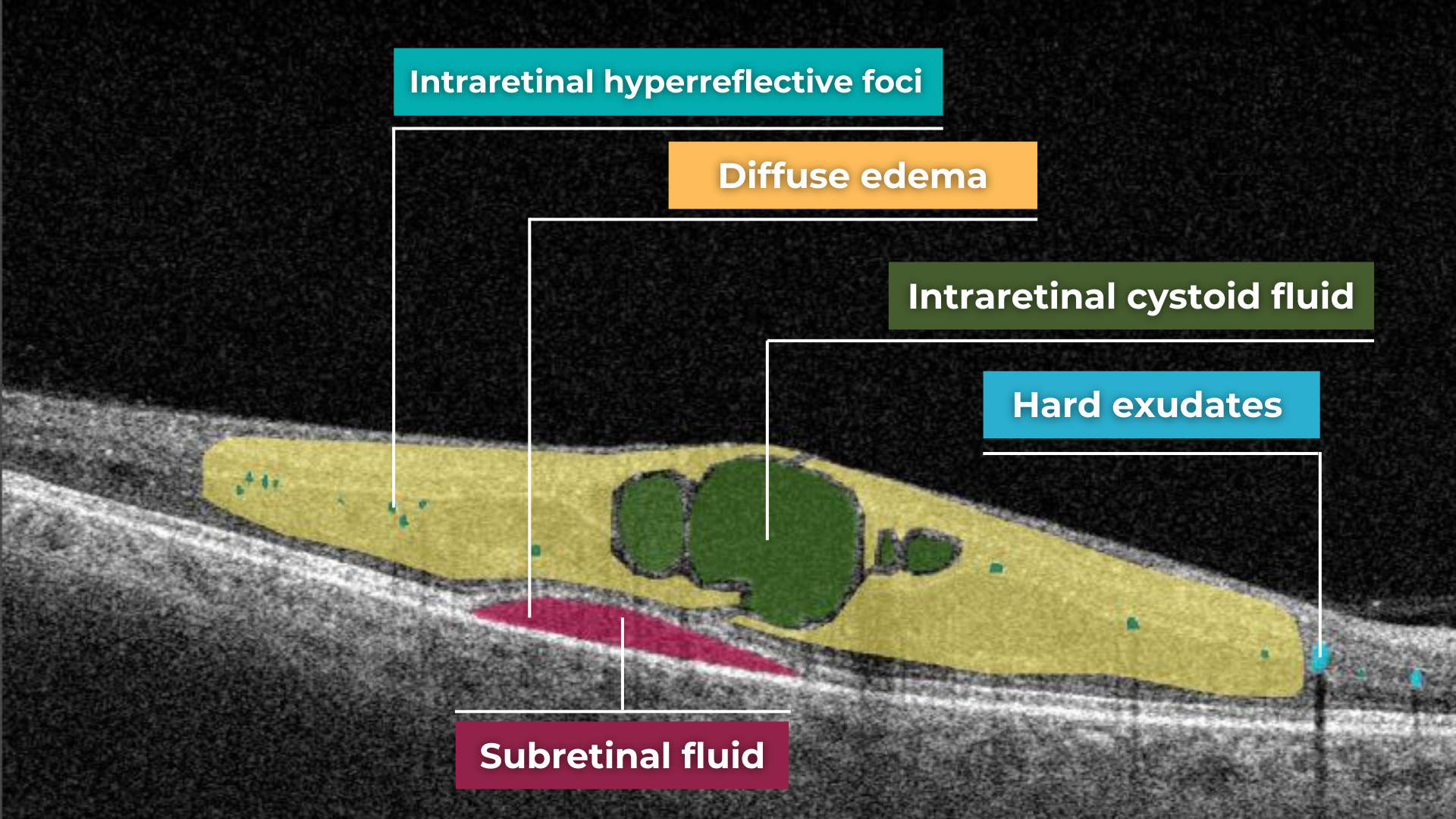
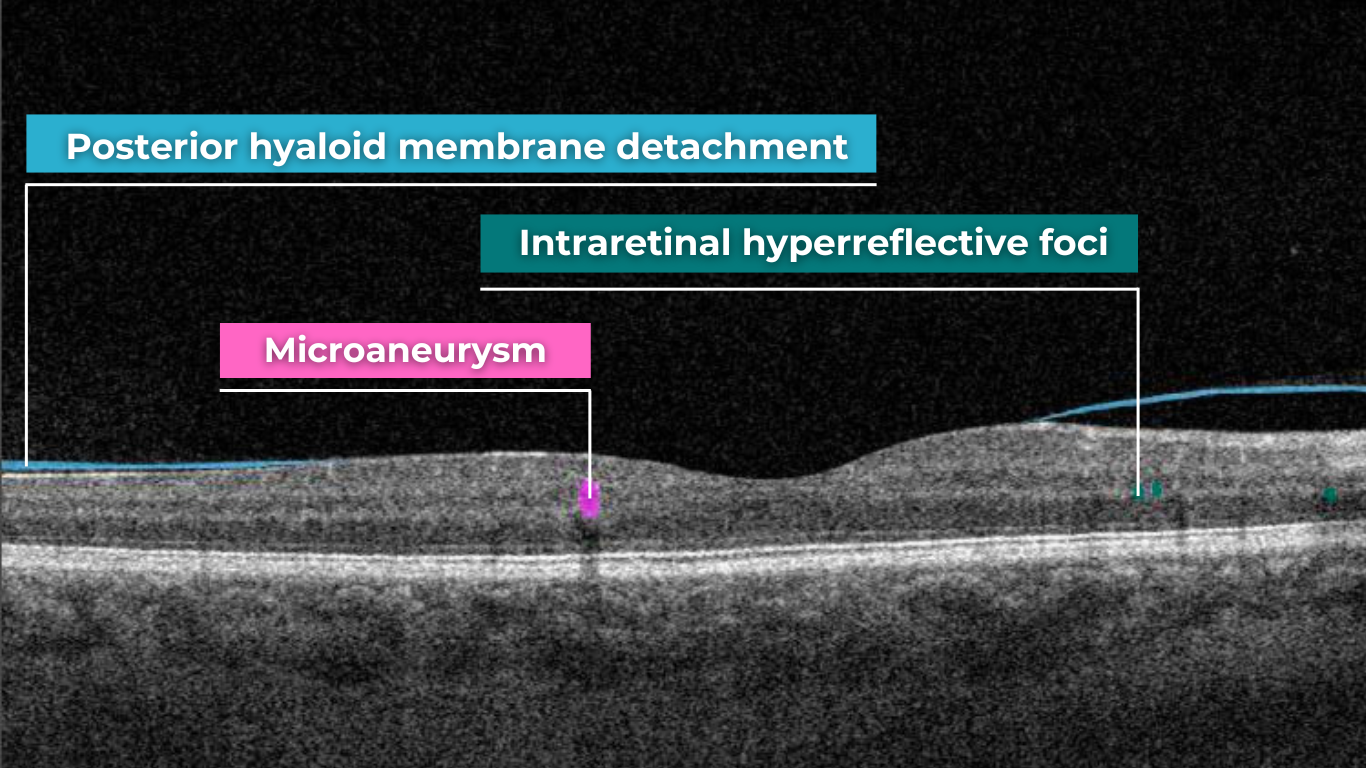
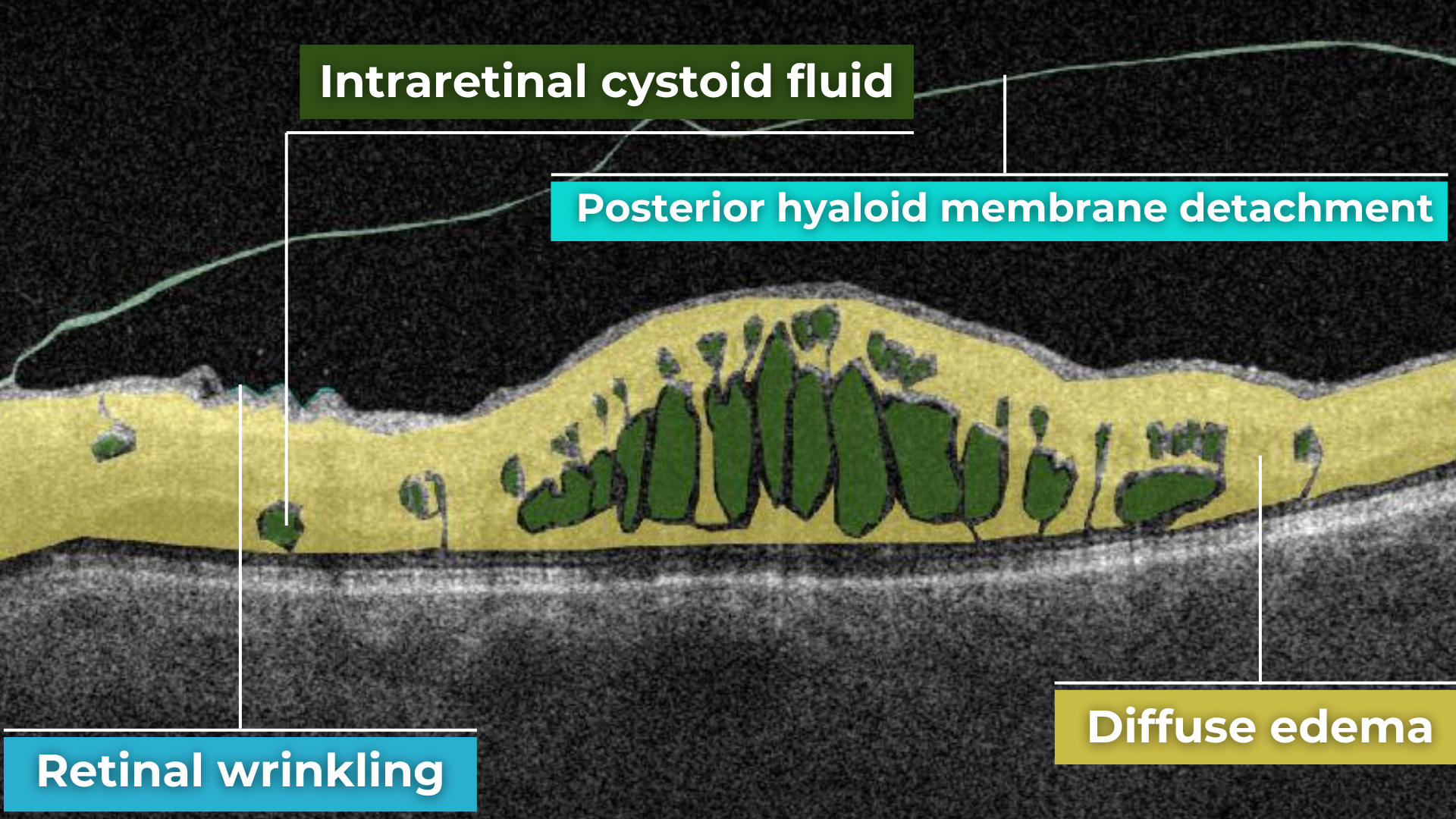
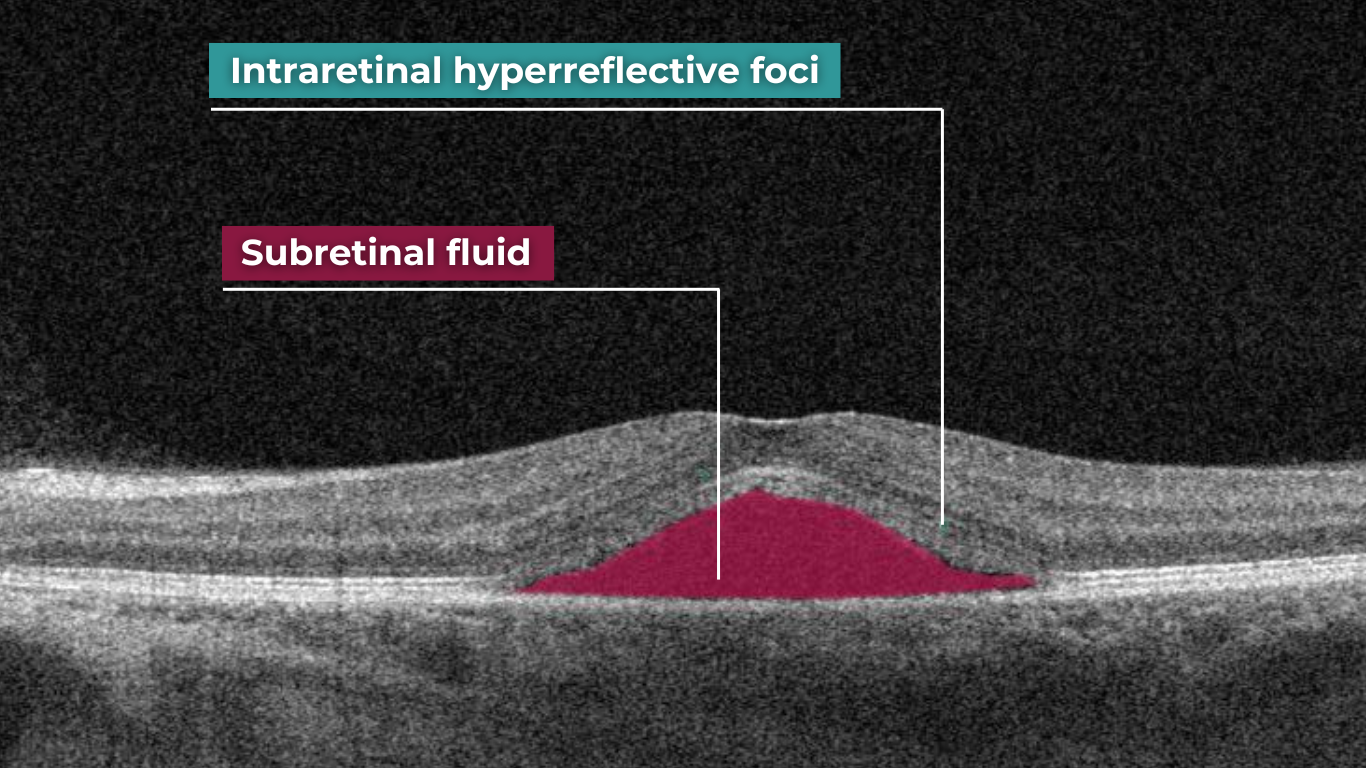
The presence of fluids such as Intraretinal Cystoid Fluid (IRC), Diffuse Edema, Subretinal Fluid (SRF), and Serous Retinal Pigment Epithelium (RPE) Detachment are critical biomarkers for conditions like nAMD, DME, DR, and RVO. Accurate detection, quantification, and tracking of these fluids are essential for monitoring disease activity, evaluating treatment efficacy, and making informed prognoses.
We created specialized more detailed functions which detect these biomarkers for more specific and accurate tracking. The AI algorithm was additionally trained to work directly with fluids taking into account the importance of these biomarkers for accurate diagnostics.
Altris AI’s advanced algorithms, trained on millions of OCT scans, provide precise and objective fluid analysis. Each of the four fluid types is localized and color-coded for clarity. Quantitative metrics such as volume, area, and ETDRS grids (1, 3, and 6 mm) are calculated and presented in mm3 or nanoliters for comprehensive evaluation. The Progression Tracking feature offers historical trend analysis with intuitive visualizations through graphs and percentages. For instance, if Cystoid Fluid (IRC) increases in volume, ECPs can immediately identify and address the change.

Precision in Geographic Atrophy (GA) Monitoring
Recent advancements in GA treatment have led to a growing need for large-scale screening in clinical practice. However, this increased demand often means higher workloads and less time for in-depth analysis.
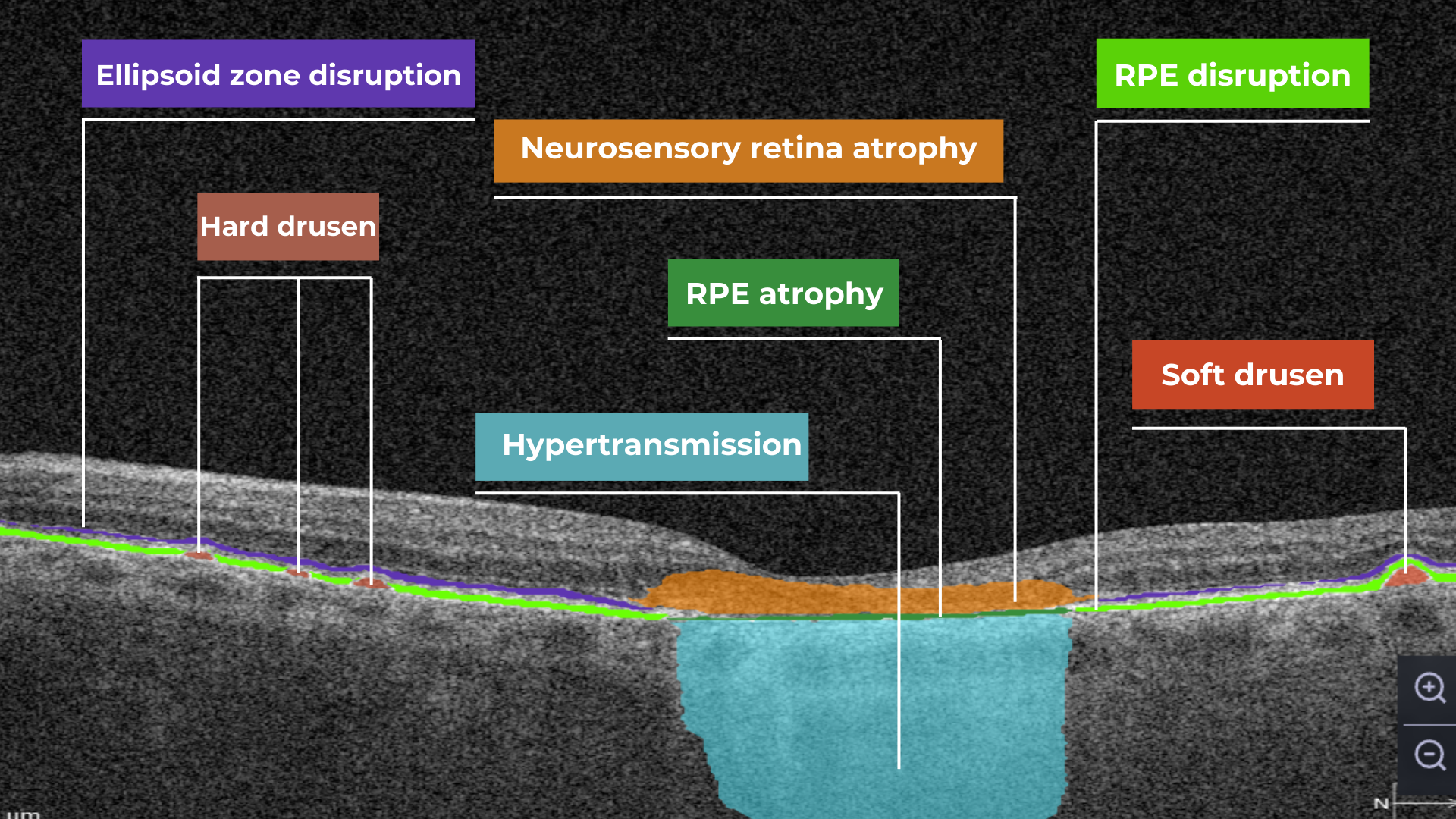
The platform facilitates automated detection, quantification, and tracking of GA by analyzing key biomarkers: Pigment Epithelium (RPE) atrophy, Hypertransmission, Neurosensory Retina Atrophy, and Ellipsoid Zone (EZ) disruption. These biomarkers are color-coded for easier identification.
We assess GA using three key criteria:
- Overlapping region of 3 biomarkers: Hypertransmission, RPE Atrophy, and Neurosensory Retina Atrophy (referred as the GA zone).
- The shortest distance from the Fovea center to the GA zone.
- Percentage of the GA zone covering the 1 mm, 3 mm, and 6 mm ETDRS grid areas.

We also improved the accuracy of a critical step in our AI pipeline: the fovea and central scan detection. Altris AI’s updated model is much more robust in detecting fovea zone and central scan now. Especially in cases when the center cannot be distinguished due to pathology presence or other reasons, the model is trained to analyze the whole surface and find reference locations from which a central scan could be determined. The new model can find an accurate center in 95% of cases, in other situations, it can efficiently estimate the center location (as opposed to a simple analysis flow used by ECPs where the geometrical center is selected). This advancement significantly enhances the precision of GA detection.
Further Progression Tracking enhances GA management by visualizing changes over time, supporting timely and accurate treatment decisions. By streamlining workflows and providing actionable insights, this feature helps ECPs make informed choices and potentially preserve vision in GA patients.
Dr. Maria Znamenska, MD, PhD, and a Chief Medical Officer at Altris AI, commented:
“We listened to our clients and introduced Fluids and GA tracking features. In 2025, eye care specialists will have the tools to combine their expertise with next-generation AI technology to effectively tackle conditions that threaten vision. Our formula is simple: detect, quantify, and track fluids, GA, and 70+ retina pathologies and biomarkers for better patient outcomes.”
About Altris AI
Altris AI is an artificial intelligence platform for OCT analysis that detects the widest range of retina pathologies and biomarkers on the market – more than 70. Leading the way in AI innovation, Altris AI provides transformative solutions that enhance the decision-making, treatment, and monitoring of retinal diseases, enabling eye care professionals to deliver exceptional patient care.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

OCT Scan Normal Eye vs 8 Most Common Pathologies
 Maria Znamenska
31.10.202414 min read
Maria Znamenska
31.10.202414 min readOCT Scan Normal Eye vs. 8 Most Common Pathologies
Differentiating between an OCT scan of a normal eye vs. a pathological one is a practical skill gained after years and years of practice. However, educating yourself on the basic differences will speed up the process. Understanding the “why” and “how” behind any changes on the OCT scan, compared to a normal macula OCT, will speed up your learning curve and deepen your expertise as a retinal expert.
The article’s first part focuses on key OCT features and their meaning as a structural change for retinal architecture. The second part discusses the most recognizable OCT features of eight common pathologies.
OCT Scan: Normal Eye
When evaluating an OCT scan, the most logical step is to understand how a normal macula OCT should look. The most telling feature across all scans is the contrast between light and dark areas. Typically, the nerve fiber layer and the underlying ganglion cell layer appear brighter than the densely packed nuclear layers. This is followed by the inner plexiform layer interface, which presents as a bright, hyperreflective area.
The inner nuclear layer, composed of densely packed nuclei, appears dark. This is followed by the outer plexiform layer, the outer nuclear layer, and Henle’s layer. The external limiting membrane, an important landmark for assessing retinal health, is also visible. The ellipsoid zone (EZ) is another bright layer, while the interdigitation zone may not always be distinguishable from the underlying RPE layer, even in healthy eyes. Finally, the RPE and inner choroid appear hyperreflective.

Structure
The ELM and EZ are critical structures to assess. In a normal macula OCT, the distance between the EZ and ELM is shorter than between the EZ and the RPE. The apparent “elevation” of the EZ in the foveal center results from the elongated outer segments of the foveal cones.
It’s important to remember that not all retinal structures are readily visible on a normal macula OCT. For example, Henle’s fiber layer is more easily distinguished in the presence of retinal pathology, such as swelling or thinning. Similarly, Bruch’s membrane is usually not visualized unless there is a separation between the RPE and Bruch’s membrane, often indicative of disease.
Thickness
Choroidal thickness is another key factor in OCT assessment. A general rule of thumb is that the choroid (between the RPE and the outer choroidal boundary) is approximately as thick as the retina. Thinning of the choroid may be observed in myopic or older patients, while marked choroidal thickening can raise suspicion for diseases like central serous retinopathy.
The OCT scan also provides information about laterality. The nerve fiber layer is characteristically thicker near the optic nerve head. Conversely, if the nerve fiber layer is not visualized in its expected location on an otherwise OCT normal scan, it could signal significant nerve fiber layer loss, potentially due to glaucoma or other optic neuropathies.
Reflectivity
Specific OCT terminology helps describe scans and differentiate normal findings from pathology.
Two fundamental concepts in OCT interpretation are hyporeflectivity and hyperreflectivity, which form the basis for understanding the structural composition of the retina as visualized in an OCT scan.
Hyporeflectivity refers to the increased light transmission capacity of a structure. The OCT scanning laser beam passes through hyporeflective structures with minimal reflection. The quintessential example of a hyporeflective structure is the vitreous humor. It appears as a dark area in the uppermost portion of a normal OCT scan, situated above the retina.
But hyporeflectivity can also be pathological, deviating from the patterns observed in a normal macula OCT; in the retina, it manifests in three primary ways.
Like the vitreous, subretinal fluid exhibits high light transmission and appears black on OCT. A uniformly black region suggests the fluid lacks cellular debris or other inclusions.

Subretinal fluid on OCT
Fluid can also accumulate within the retinal layers, for example, between the layers of the neuroepithelium. This intraretinal fluid also appears hyporeflective on OCT.

Intraretinal fluid on OCT
Following a degenerative process within the retina, a cavity or void may form where retinal tissue has been lost. These degenerative cavities lack the cellular components necessary to reflect light and thus appear as dark spaces on OCT. It’s important to differentiate these cavities from cystic spaces, which may have distinct clinical implications.
One example is outer retinal tubulations. While associated with various diseases, outer retinal tubulations (ORTs) generally indicate outer retinal degeneration and atrophy.

Outer retinal tubulations on OCT
Hyperreflectivity, unlike hyporeflectivity, indicates structures with high light reflectance. On the grayscale spectrum of an OCT image, hyperreflective structures appear progressively whiter.
The retinal pigment epithelium (RPE) complex and Bruch’s membrane are considered the most hyperreflective structures in a normal macula OCT.
Pathological processes can introduce new hyperreflective elements within the retina, aiding in differentiating normal and abnormal OCT scans. A typical example is hard exudates, frequently observed in diabetic retinopathy. These lipid-rich deposits are extremely dense, causing them to appear bright white on OCT due to the complete reflection of incident light. Furthermore, this high density leads to a shadowing effect beneath the deposits, caused by strong backscattering of the OCT signal.

Hard exudates and shadowing on OCT
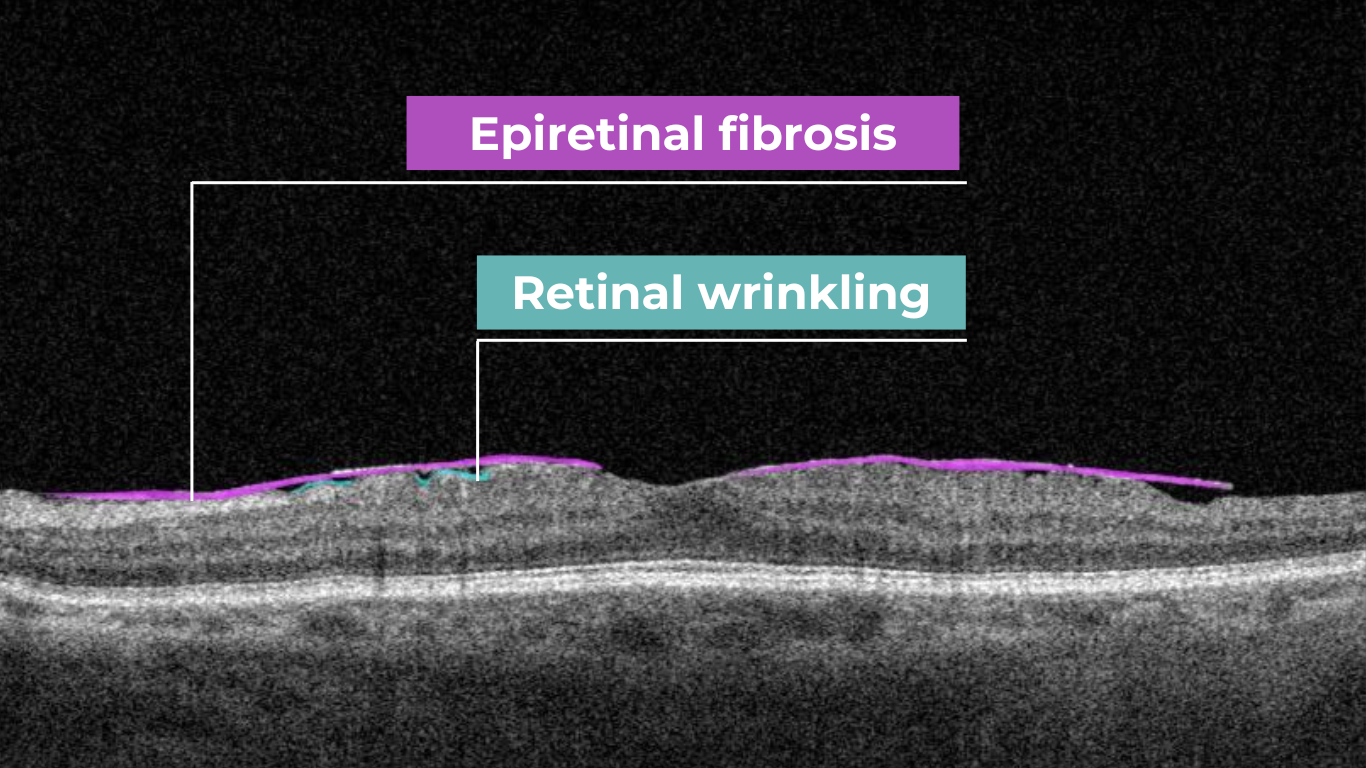
Epiretinal membranes (ERMs) – a thin membrane or layer of scar tissue that forms over the retina – are also hyperreflective. It is composed of dense connective tissue with high light-reflecting properties and appears white on OCT scans.
Integrity
Beyond hypo- and hyperreflectivity, OCT interpretation involves assessing the structural integrity of retinal layers. For instance, in an OCT scan of a normal eye, Bruch’s membrane appears as a thin, continuous line underlying the retinal pigment epithelium (RPE). The RPE is a monolayer of cells, ideally presenting with a smooth and uniform optical density. However, some pathologies, particularly early stages of age-related macular degeneration (AMD), may show unevenness or integrity loss in the RPE and Bruch’s membrane complex.
Disruption of the ellipsoid zone (EZ) is a particularly concerning finding on OCT, often indicating photoreceptor damage. Significant disruption of the EZ in the central macula is a strong biomarker for adverse visual outcomes.
The closer the loss of integrity extends toward the foveal center, the poorer the visual prognosis tends to be.

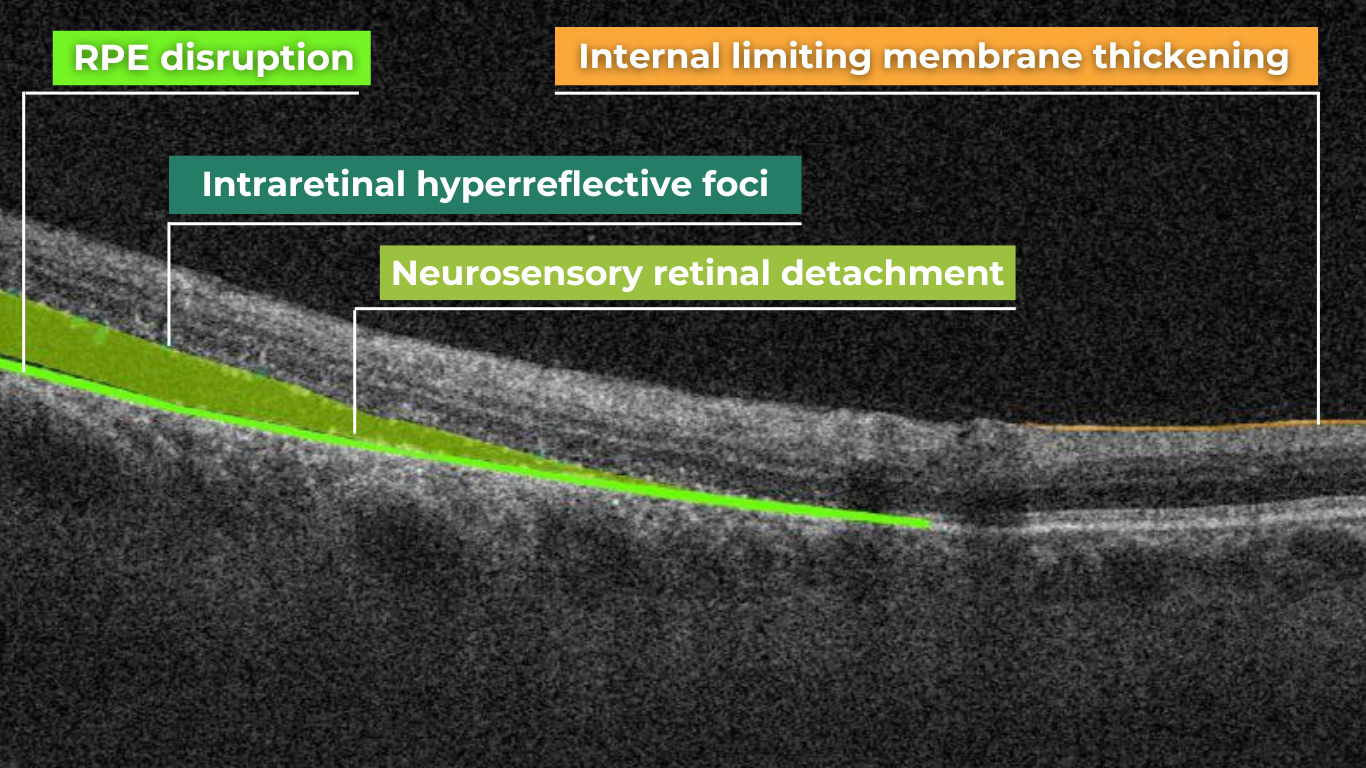
Ellipsoid zone disruption on OCT
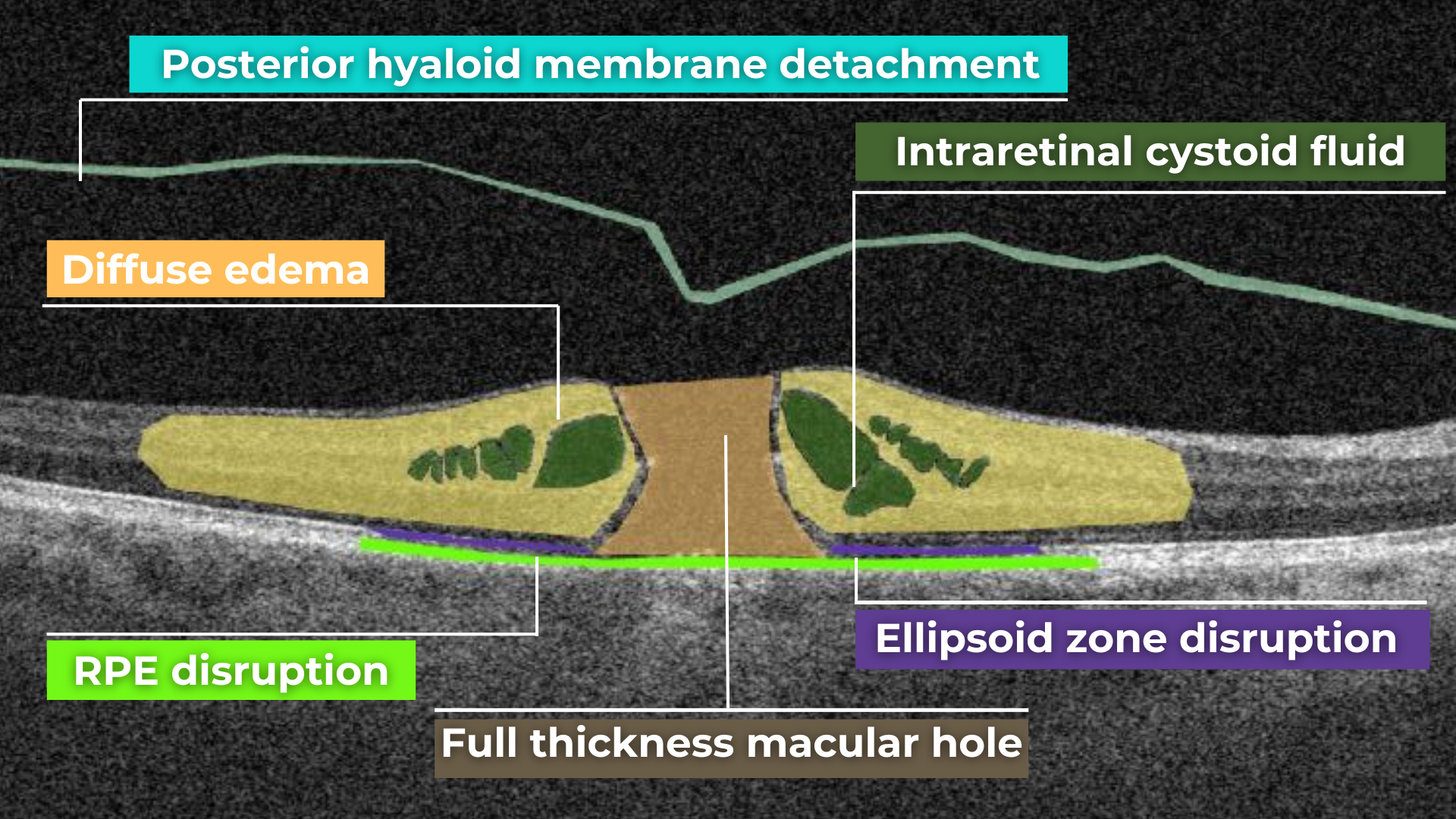
OCT also plays a crucial role in visualizing and characterizing breaks in the structural integrity of the retina. These breaks, commonly referred to as retinal tears or holes, can be classified as full-thickness or partial-thickness, depending on the extent of retinal involvement.
Full-thickness breaks completely separate all retinal layers, while partial-thickness breaks involve only some retinal layers. OCT allows for precise delineation of the layers involved and the overall morphology of the break.
Retinal holes can also be categorized by their location. Macular holes, as the name suggests, involve the central retina and can lead to significant central vision loss and require prompt attention.

Lamellar macular hole on OCT
Non-macular holes occur outside the central macular region, often in the peripheral retina. While they may not cause immediate central vision disturbances, they can still lead to serious complications, such as retinal detachment, if left untreated.
Definition
The blurring of retinal structures, or loss of definition, is another key OCT concept. This loss of the retina’s normal layered organization, seen in diseases like AMD, manifests as indistinct layers merging into a homogenous mass.

Disorganisation of retinal inner layers on OCT
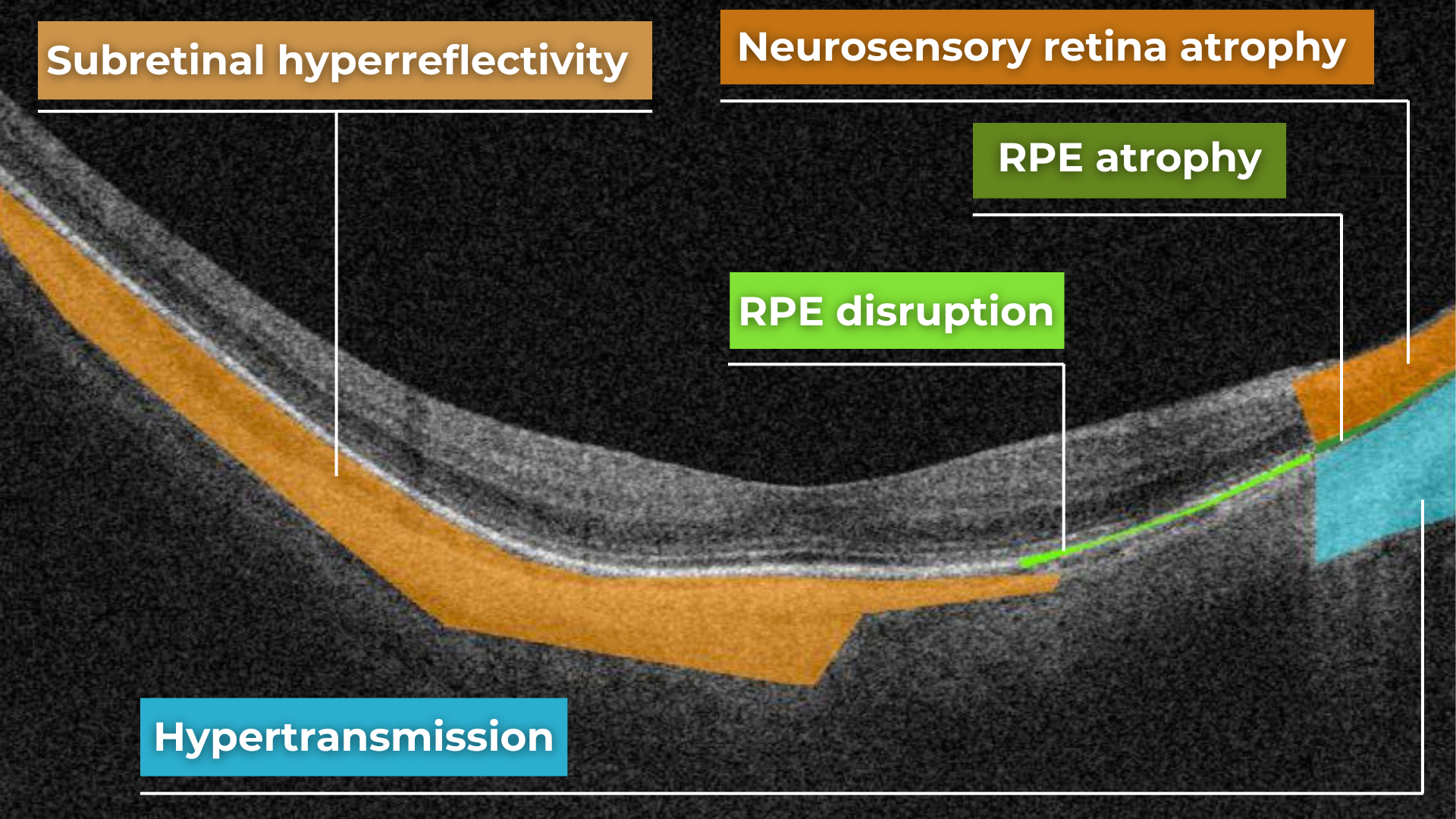
Hypertransmission in OCT refers to enhanced signal penetration due to reduced blockage of the OCT light signal. This phenomenon is frequently observed in geographic atrophy, a late stage of AMD characterized by the atrophy of the retinal pigment epithelium, choriocapillaris, and photoreceptors.
 Hypertransmission on OCT
Hypertransmission on OCTIn a normal macula OCT, a signal is attenuated as it traverses the various retinal layers, with a portion of the signal being reflected to the detector. However, in geographic atrophy (GA), the loss of RPE and other retinal structures reduces this attenuation, allowing the OCT signal to penetrate deeper into the choroid. This increased penetration results in a stronger signal return from the choroidal layers, creating essentially a “corridor” of enhanced signal penetration through the atrophic areas of the retina. This deep penetration and strong signal return, unfortunately, indicate significant retinal damage and are associated with a poor visual prognosis.
Displacement
Another term used to describe OCT scan results is elevation. It refers to the upward displacement of retinal structures from their normal anatomical position. In the context of age-related macular degeneration (AMD), elevation is frequently associated with the presence of drusen.
Drusen are extracellular deposits that accumulate between the retinal pigment epithelium (RPE) and Bruch’s membrane. They are a hallmark of AMD and can vary in size, shape, and composition. Drusen are typically categorized as hard, soft, or confluent based on their ophthalmoscopic appearance.

Hard and soft drusen on OCT
In contrast to elevation, depression in OCT describes the inward displacement or concavity of retinal structures. This can be a manifestation of various pathological processes, with a prominent example of degenerative myopia.

Degenerative myopia on OCT
OCT scan: normal eye transformation through pathologies
Age-related macular degeneration (AMD)
AMD is an acquired degenerative macular disease usually affecting individuals over the age of 55 years. It is characterized by pathologic alterations of the outer retina, retinal pigment epithelium (RPE), Bruch’s membrane, and choriocapillaris complex, including drusen formation and pigmentary changes.
AMD is a progressive disease, and in advanced stages, central geographic atrophy and neovascularization, may develop and reduce vision. OCT plays a critical role in distinguishing between the different stages and forms of AMD, particularly when compared to the features of an OCT normal scan.
Wet AMD

Neovascular or “wet” age-related macular degeneration (nAMD) arises from the aberrant growth of choroidal vessels that penetrate Bruch’s membrane and invade the subretinal space. These abnormal vessels leak fluid and blood, disrupting the retinal architecture and causing vision loss.
Several key OCT features can signal the presence and activity of nAMD in comparison to a normal OCT scan:
- Fluid Accumulation: The presence and location of fluid are hallmarks of nAMD (hence the term ‘wet AMD’). Intraretinal fluid, appearing within the retinal layers, often signifies more severe disease and a poorer visual prognosis than subretinal fluid, which accumulates beneath the retina.
- RPE Detachment: Serous PED appears as a dome-shaped elevation of the RPE due to fluid accumulation beneath it. PEDs often accompany nAMD and can vary in size and shape.
- Disruption of Retinal Layers: nAMD can disrupt the normal retinal architecture, particularly the photoreceptor layer. Damage to the ellipsoid zone (EZ) and external limiting membrane (ELM) is visible on OCT and correlates with visual impairment.
- Hyperreflective Foci: Hyperreflective dots (HRDs) are small, bright spots scattered throughout the retina.
- Subretinal Hyperreflective Material (SHRM): Appears as a hyperreflective band between the retina and RPE. Its composition varies but may include fluid, fibrin, blood, and neovascular tissue; it can be associated with poorer visual outcomes.
- RPE Tears: These are disruptions in the RPE monolayer, often occurring in areas of PED. RPE tears can lead to significant vision loss and are an important complication of nAMD.
- Choroidal Changes: nAMD can also affect the choroid, the vascular layer beneath the RPE.
Dry AMD

In its early stages, Dry AMD is characterized by drusen and pigmentary abnormalities resulting from alterations in the retinal pigment epithelium (RPE). Later, it can progress to geographic atrophy (GA) or outer retinal atrophy.
The three classic findings in Dry AMD are drusen, pigmentary changes, and geographic atrophy.
Drusen are classified as:
- small (<65 um),
- medium (65 – 124 um),
- or large (>125 um).
While both drusen and pigmentary changes can appear as yellowish deposits in the retina, pigmentary changes are often more varied in color (ranging from yellow to brown or black) and less defined in shape than the generally circular drusen.
Geographic atrophy typically begins in the paracentral macula, often surrounding the fovea in a horseshoe pattern. It can eventually involve the fovea itself, leading to severe vision loss.
Diabetic Retinopaty (DR)

Diabetic retinopathy (DR), a leading cause of vision loss in working-age populations, is characterized by retinal vascular abnormalities. It progresses from non-proliferative DR (NPDR), marked by vascular leakage and capillary occlusion, to proliferative DR (PDR), where neovascularization can lead to severe vision impairment through vitreous hemorrhage or retinal detachment.
OCT can aid in identifying the earliest sign of DR: microaneurysms. They appear as small, distinct, oval-shaped, hyperreflective, walled structures associated with microvascular damage. Specifically, the structural weakness of the vessel wall of MAs causes fluid leakage, resulting in edema.

Another consequence of microaneurysm formation is the progression to intraretinal hemorrhages (IRH), often called ‘dot-blot’ hemorrhages. These appear as hyperreflective foci on OCT cross-sections, with varying degrees of opacification.
Diabetic macular edema (DME) can occur at any stage of the disease and is the most common cause of vision loss in those with diabetes. It results from a blood-retinal barrier breakdown, leading to fluid leakage and retinal thickening.
Retinal vein occlusions

Retinal vein occlusions (RVOs) are blockages of the retinal veins responsible for draining blood from the retina. These blockages can affect either the central retinal vein (CRVO) or one of its branches (BRVO). RVOs are more prevalent in older individuals and those with underlying vascular conditions such as high blood pressure, high cholesterol, a history of heart attack or stroke, diabetes, or glaucoma. The primary vision-threatening complications of RVO are macular edema, which involves fluid accumulation in the central retina, and retinal ischemia, which results from insufficient blood flow to the retina.
While both Central Retinal Vein Occlusion (CRVO) and Branch Retinal Vein Occlusion (BRVO) involve blockage of a retinal vein, the underlying cause and location of the blockage differ.
CRVO occurs when a thrombus (blood clot) blocks the central retinal vein near the lamina cribrosa, where the optic nerve exits the eye.
In contrast, BRVO typically occurs at an arteriovenous crossing point, where a retinal artery and vein intersect. Atherosclerosis (hardening of the arteries) can compress the vein at this crossing point, leading to thrombus formation and occlusion.
In CRVO, the retina often exhibits extensive intraretinal hemorrhages, dilated and tortuous veins, and cotton-wool spots. This constellation of findings is classically described as a “blood and thunder” appearance. In BRVO, the signs are typically localized to the area of the retina drained by the affected vein. Macular edema, characterized by retinal thickening and cystoid spaces within the retina, is a common finding in CRVO and BRVO and can significantly contribute to vision loss.
Central serous retinopathy

Central serous chorioretinopathy (CSCR) is a common retinal disorder that causes visual impairment and altered visual function. It is classified as a pachychoroid disease, including conditions like polypoidal choroidal vasculopathy and pachychoroid neovasculopathy.
OCT imaging in CSCR often reveals a thicker-than-average choroid.
This diagnostic is particularly useful in cases where clinical examination findings are inconclusive, distinguishing subtle differences between normal and abnormal OCT scans in terms of structural changes, such as small pigment epithelial detachments (PEDs) and hyperreflective subretinal fluid, that may not readily appear on clinical exams.
Furthermore, OCT is valuable for monitoring disease progression and resolution in chronic CSCR cases. A distinguishing feature that can also be seen in CSR is the appearance of the retinal pigment epithelium: the RPE line typically appears straight in non-affected areas, while it can appear wavy or irregular in areas with CSCR.
Epiretinal membrane (Epiretinal fibrosis)

Epiretinal fibrosis (epiretinal membrane/macular pucker) is a common condition affecting the central retina, specifically the macula. It is characterized by a semi-translucent, avascular membrane that forms on the retinal surface, overlying the internal limiting membrane (ILM), which is absent on a normal macula OCT.
OCT plays a crucial role in assessing the severity of ERMs, revealing the extent of macular distortion and the involvement of retinal layers.
OCT findings in ERMs are used to stage the severity of the membrane, ranging:
- Stage 1: ERMs are mild and thin. Foveal depression is present.
- Stage 2: ERMs with widening the outer nuclear layer and losing the foveal depression.
- Stage 3: ERMs with continuous ectopic inner foveal layers crossing the entire foveal area.
- Stage 4: ERMs are thick with continuous ectopic inner foveal and disrupted retinal layers.
Retinal detachment

Retinal detachment is an important cause of decreased visual acuity and blindness, a common ocular emergency often requiring urgent treatment.
It occurs when subretinal fluid accumulates between the neurosensory retina and the retinal pigment epithelium through three mechanisms:
- Rhegmatogenous: a break in the retina allowing liquified vitreous to enter the subretinal space directly.
- Tractional: proliferative membranes on the surface of the retina or vitreous pull on the neurosensory retina, causing a physical separation between the neurosensory retina and retinal pigment epithelium
- Exudative: accumulation of subretinal fluid due to inflammatory mediators or exudation of fluid from a mass lesion/insufficient RPE function
OCT helps identify foveal status and diagnose tractional or exudative retinal detachments, aiding in treatment planning.
Macular hole

Macular holes are full-thickness defects of retinal tissue involving the anatomic fovea and primarily the foveola of the eye. They are thought to form due to anterior-posterior forces, tangential forces and weakening in the retinal architecture that result in openings in the macular center.
The International Vitreomacular Traction Study (IVTS) Group formed a classification scheme of vitreomacular traction and macular holes based on OCT findings:
- Vitreomacular adhesion (VMA): No distortion of the foveal contour; size of attachment area between hyaloid and retina defined as focal if </= 1500 microns and broad if >1500 microns
- Vitreomacular traction (VMT): Distortion of foveal contour present or intraretinal structural changes in the absence of a full-thickness macular hole; size of attachment area between hyaloid and retina defined as focal if </= 1500 microns and broad if >1500 microns.
- Full-thickness macular hole (FTMH): Full-thickness defect from the internal limiting membrane to the retinal pigment epithelium. Described 3 factors: 1) Size – horizontal diameter at narrowest point: small (≤ 250 μm), medium (250-400 μm), large (> 400 μm); 2) Cause – primary or secondary; 3) Presence of absence of VMT.
Glaucoma

Glaucoma is a progressive optic neuropathy that is multifactorial and degenerative. It is characterized by the death of retinal ganglion cells (RGCs) and their axons, leading to the characteristic optic disc and retinal nerve fiber layer (RNFL) structural changes and associated vision loss. One of the most effective ways to get information about nerve states is OCT.
The Glaucoma OCT test provides valuable information about ganglion cells: damage to the ganglion cells or their processes leads to thinning across respective layers, which we can measure as the thickness of the ganglion cell complex.
Key things to focus on when working with OCT for glaucoma detection:
- Look for thinning of the pRNFL, particularly in the inferior and superior quadrants, asymmetrical thinning between a patient’s eyes
- Assess the thickness of the ganglion cell-inner plexiform layer, macular RNFL, and the overall ganglion cell complex.
- Monitoring: Seek significant decreases over time in pRNFL thickness (≥5 μm globally, ≥7-8 μm in specific sectors) or in average GCIPL thickness (>4μm).
AI-powered OCT interpretation tools, such as Altris AI, AI for OCT, can further assist clinicians by providing automated calculations of RNFL thinning in the upper and lower hemispheres and the asymmetry levels between them.
Summing up
OCT has revolutionized ophthalmology, bringing a wealth of new details and challenges. The human eye can easily miss subtle abnormalities on complex scans, making accurate interpretation critical. While experience is essential, relying solely on “learning by doing” poses risks.
AI-powered OCT interpretation software bridges this gap, offering a safety net during the learning curve and beyond. AI-powered second opinion on OCT scans enhances diagnostic accuracy, empowers clinicians, and allows them to spend more time for a meaningful connection with patients.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.

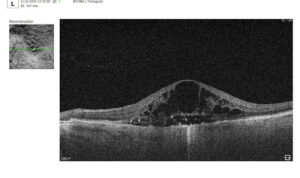
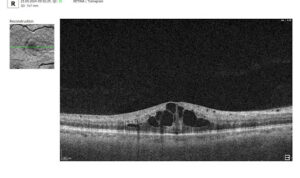


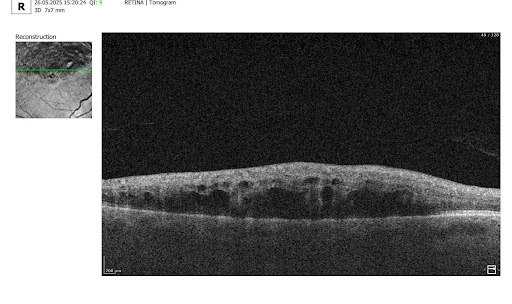
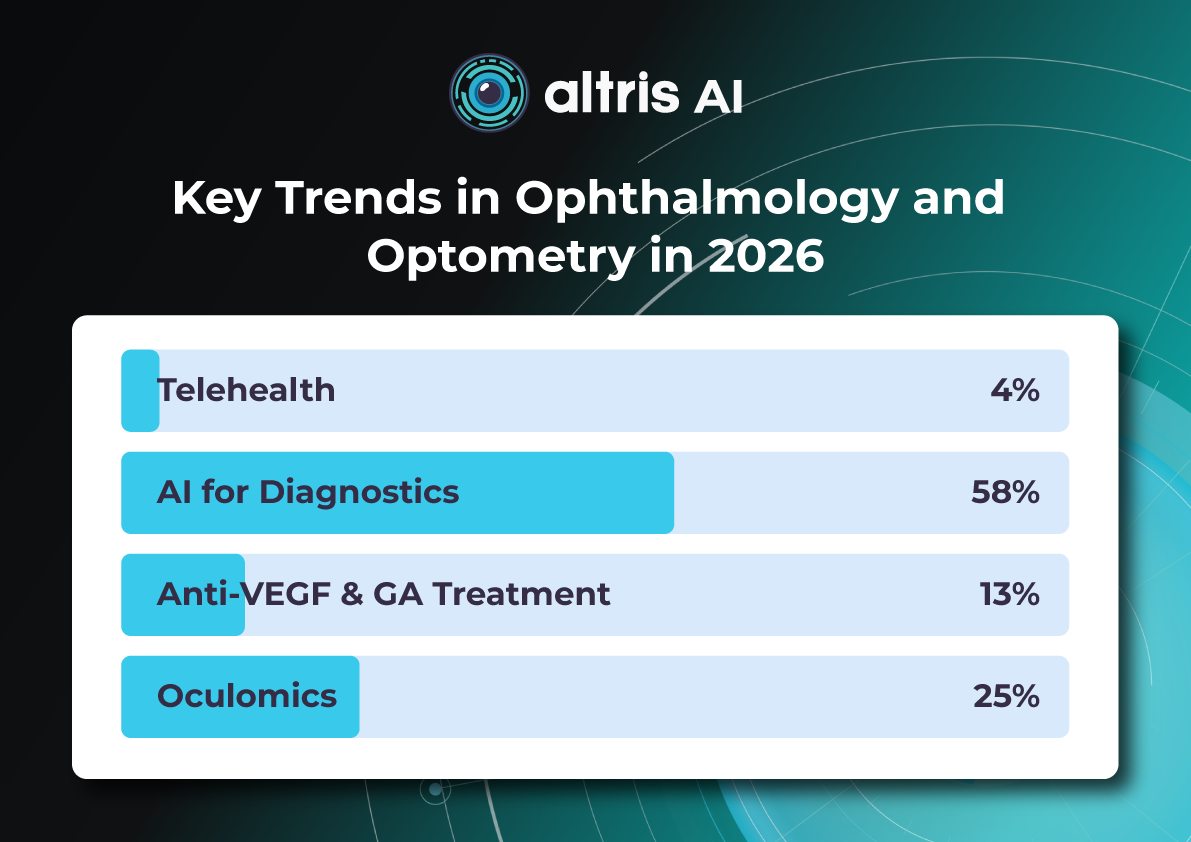







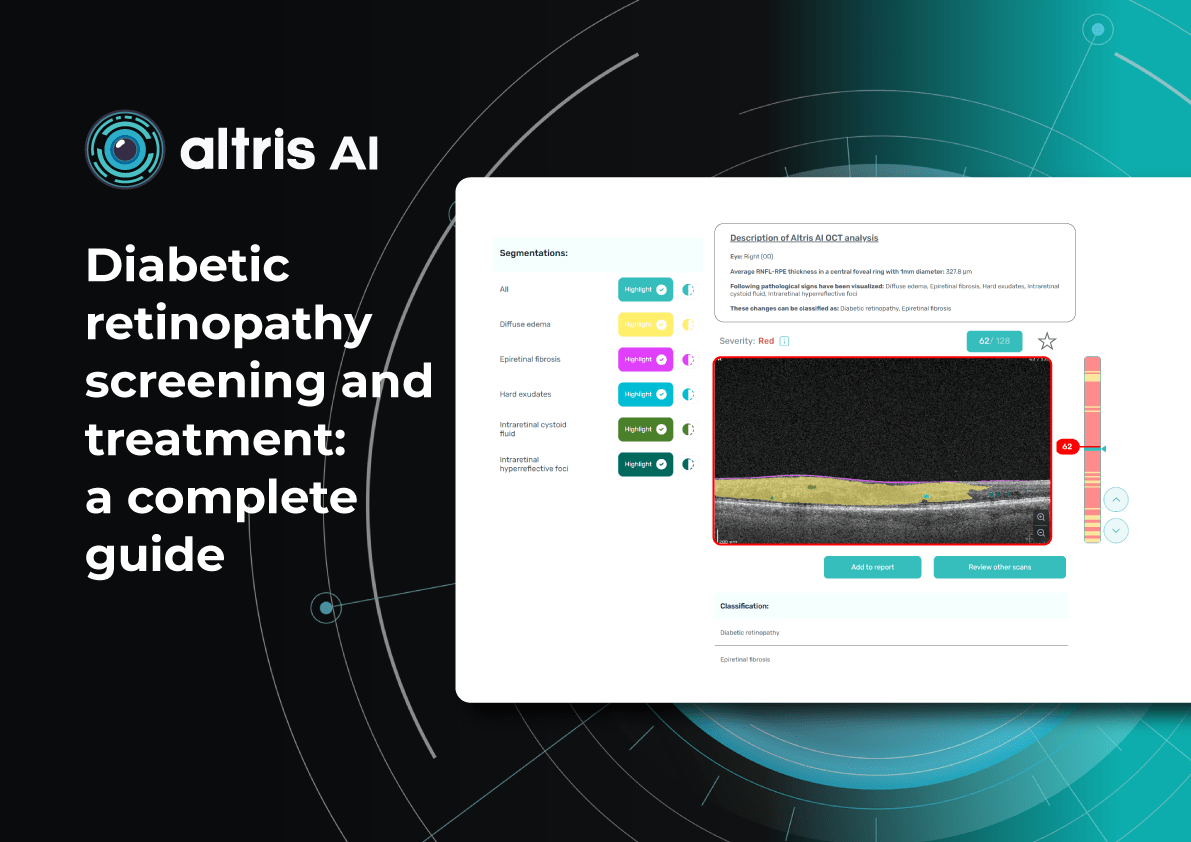

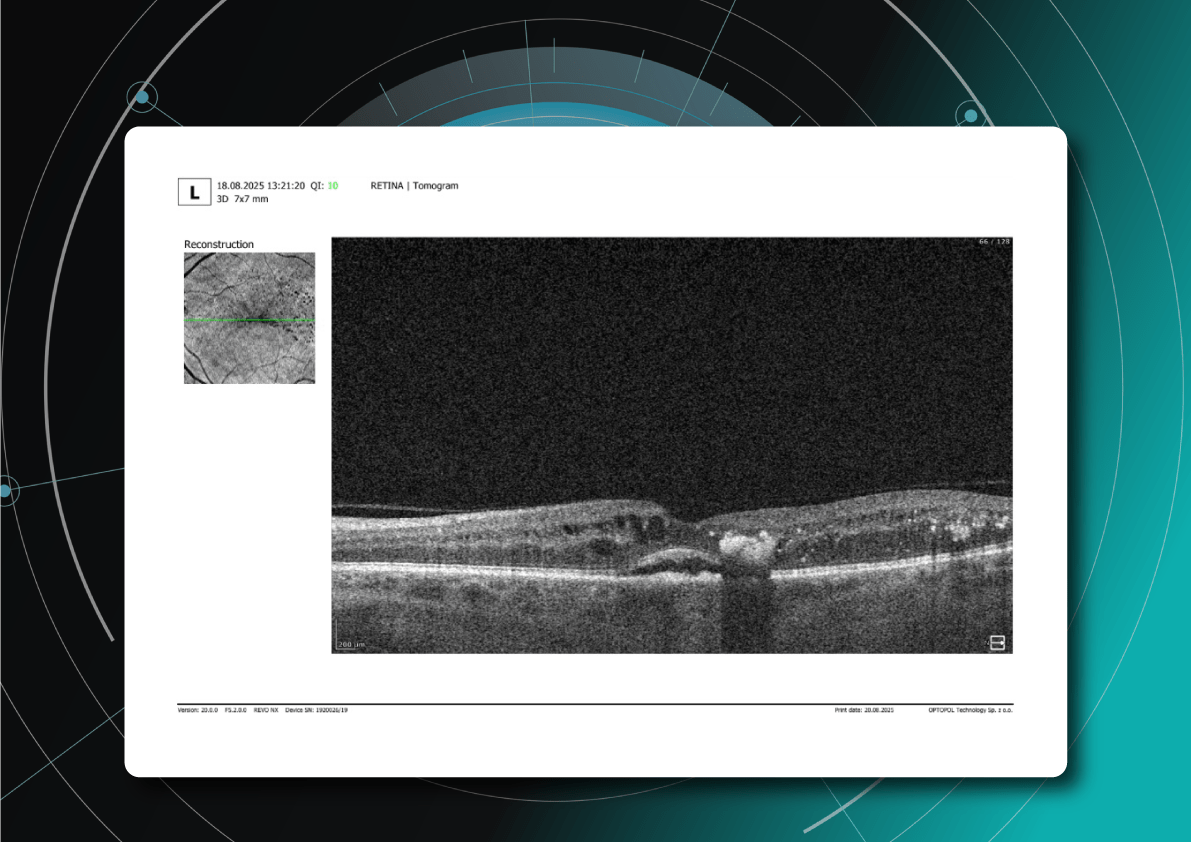


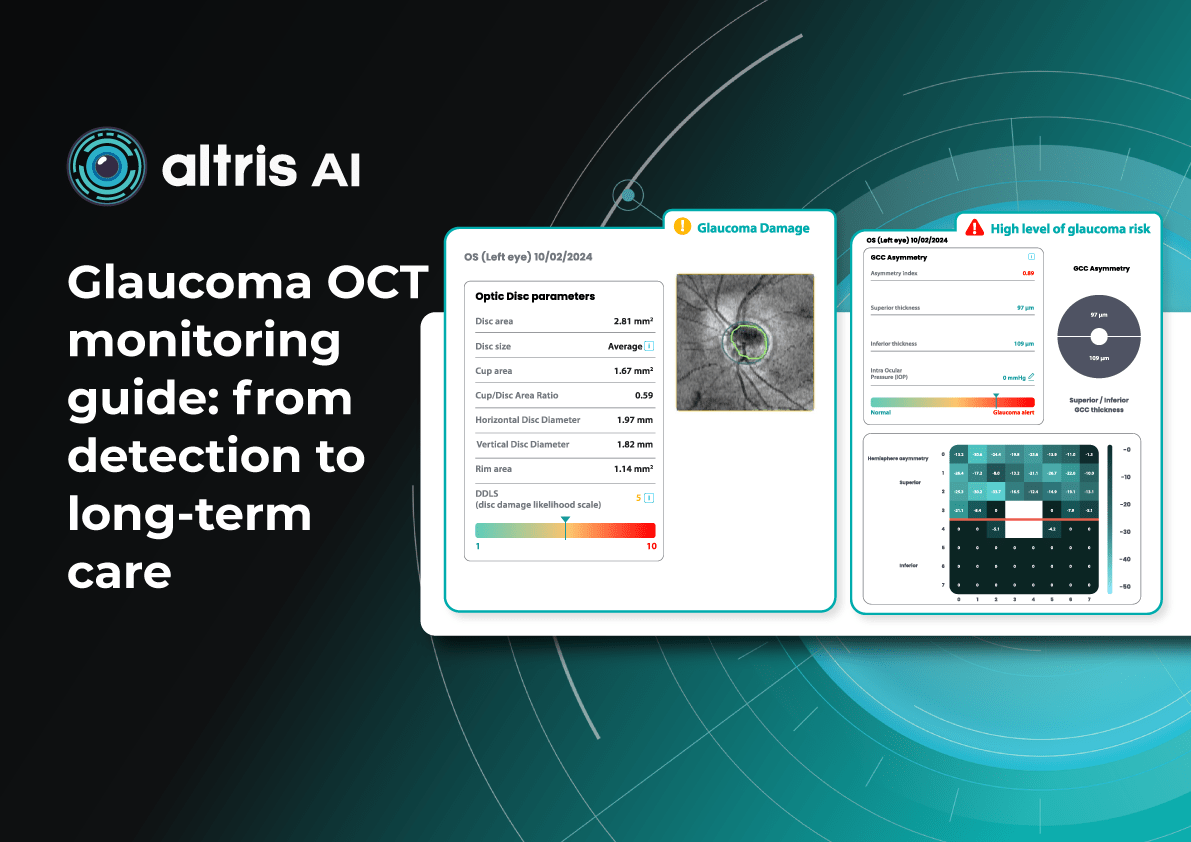
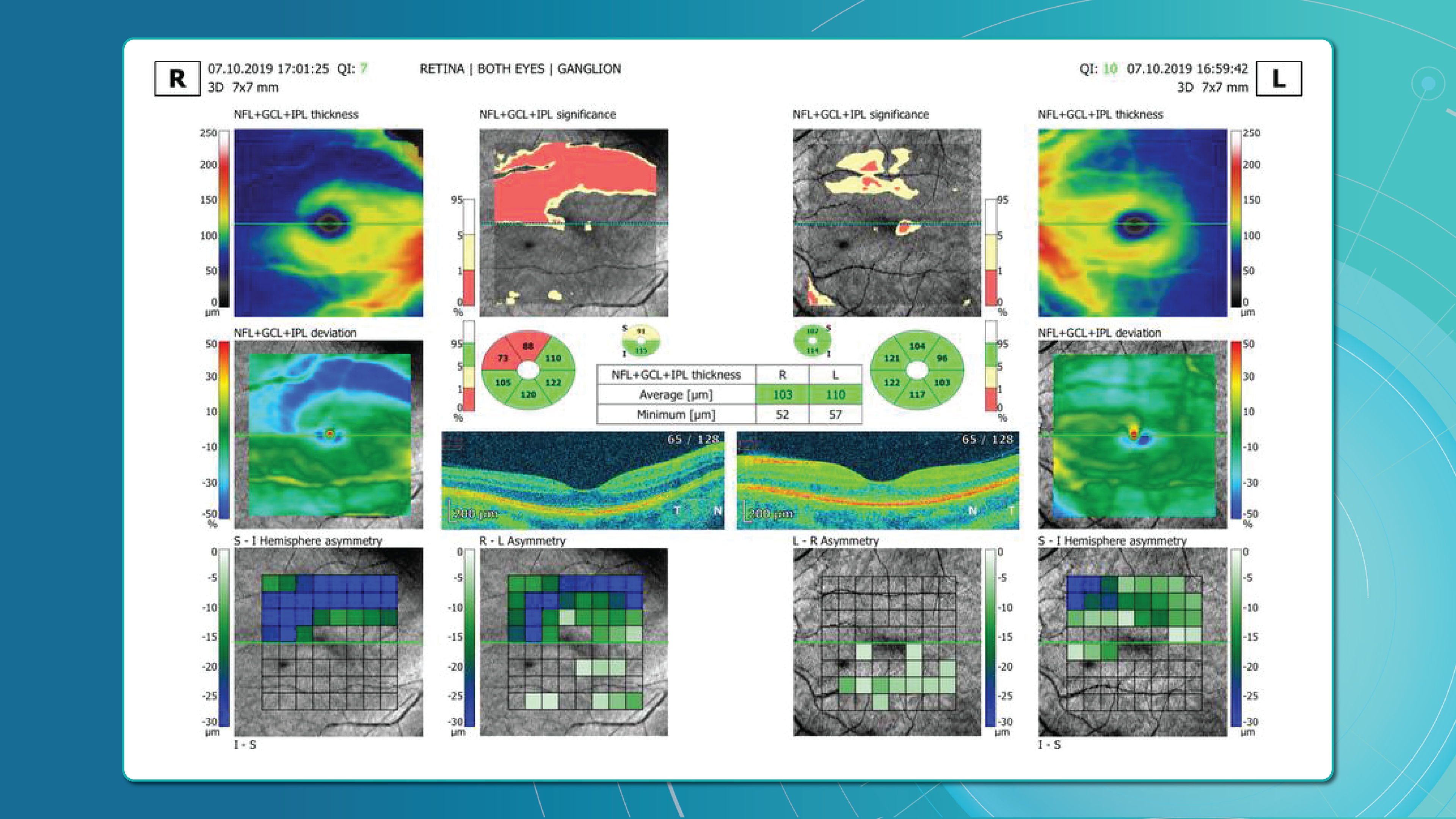
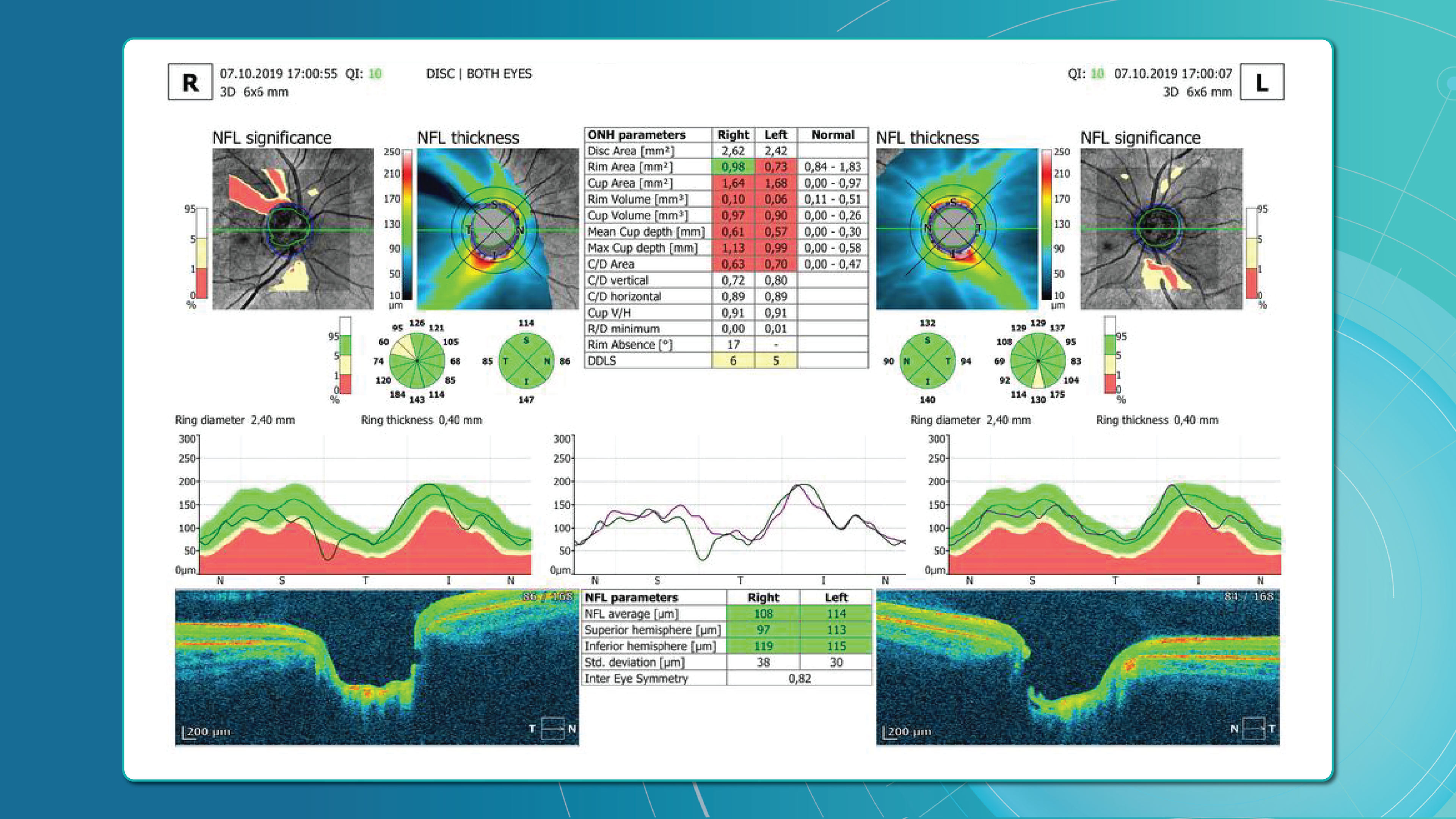
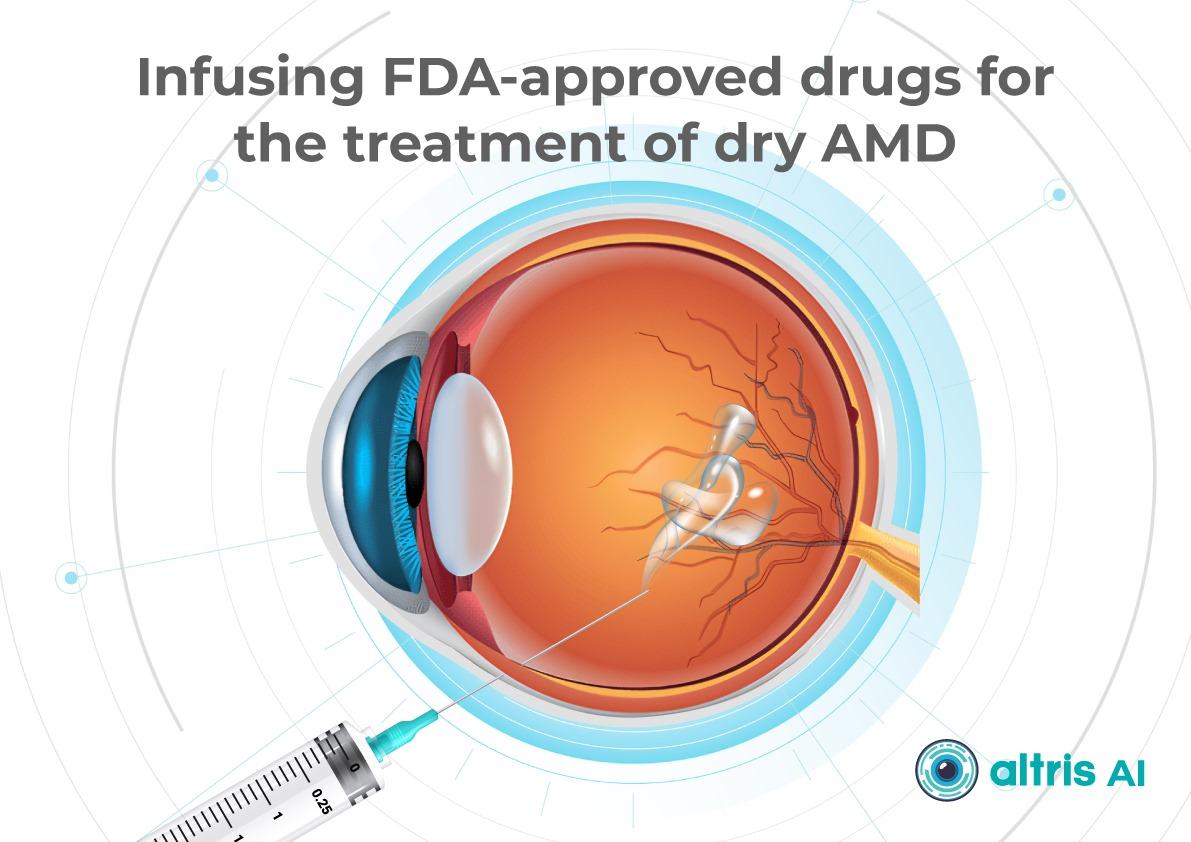








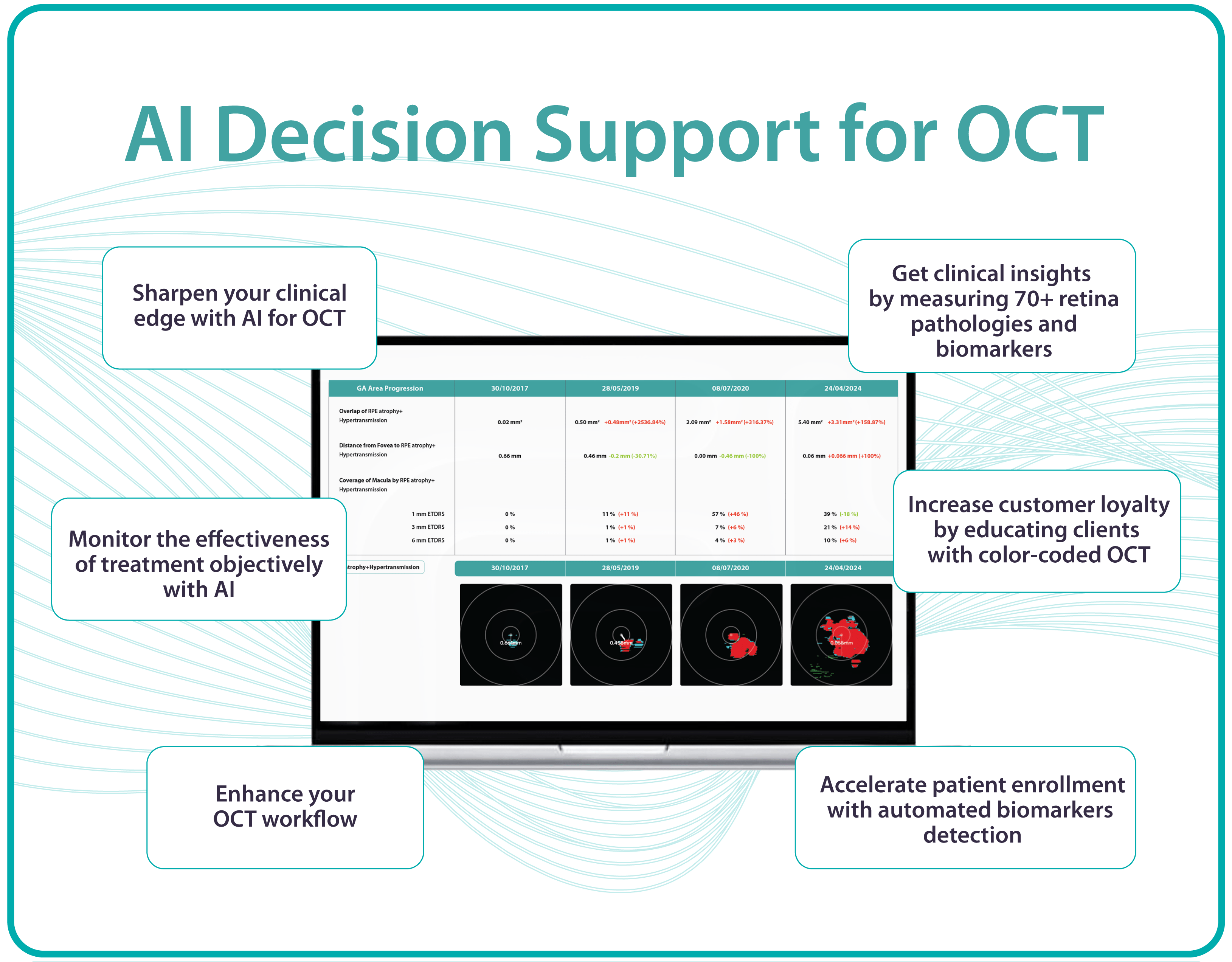

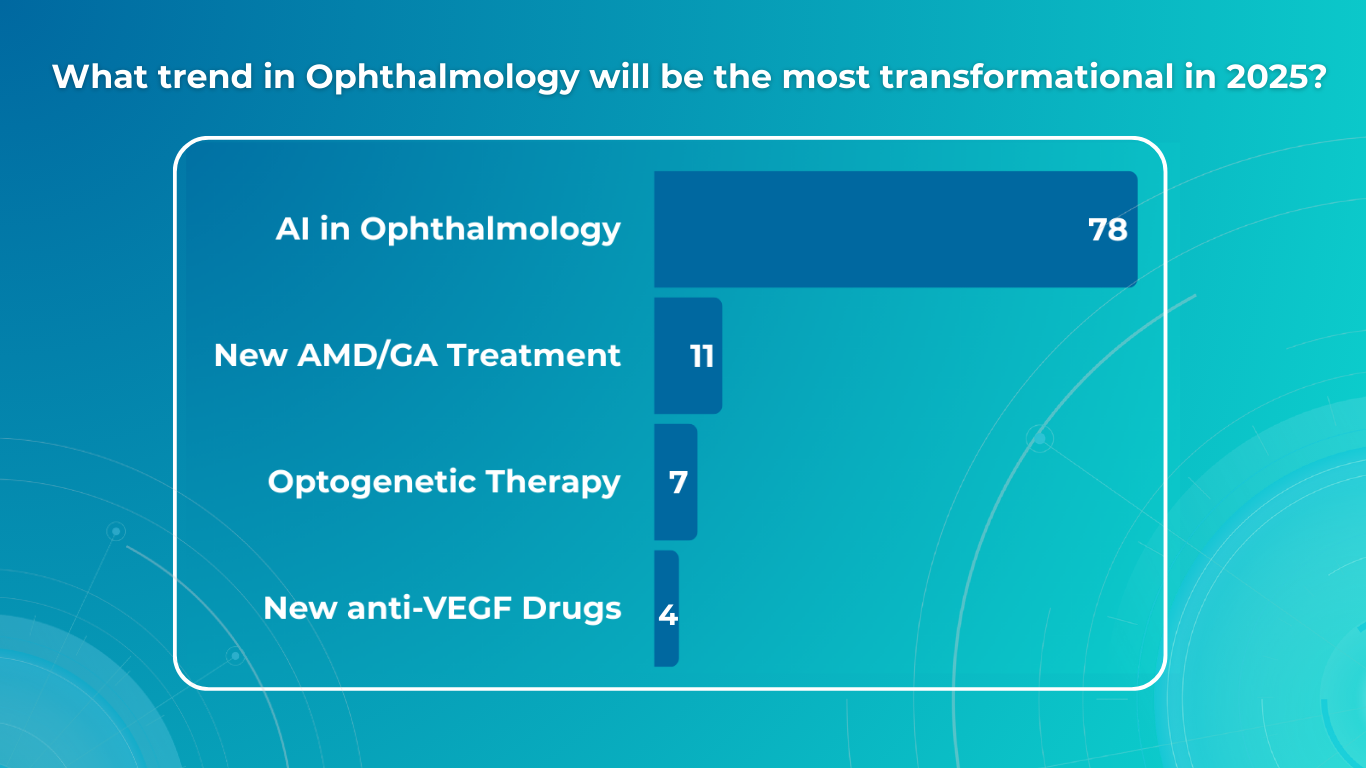











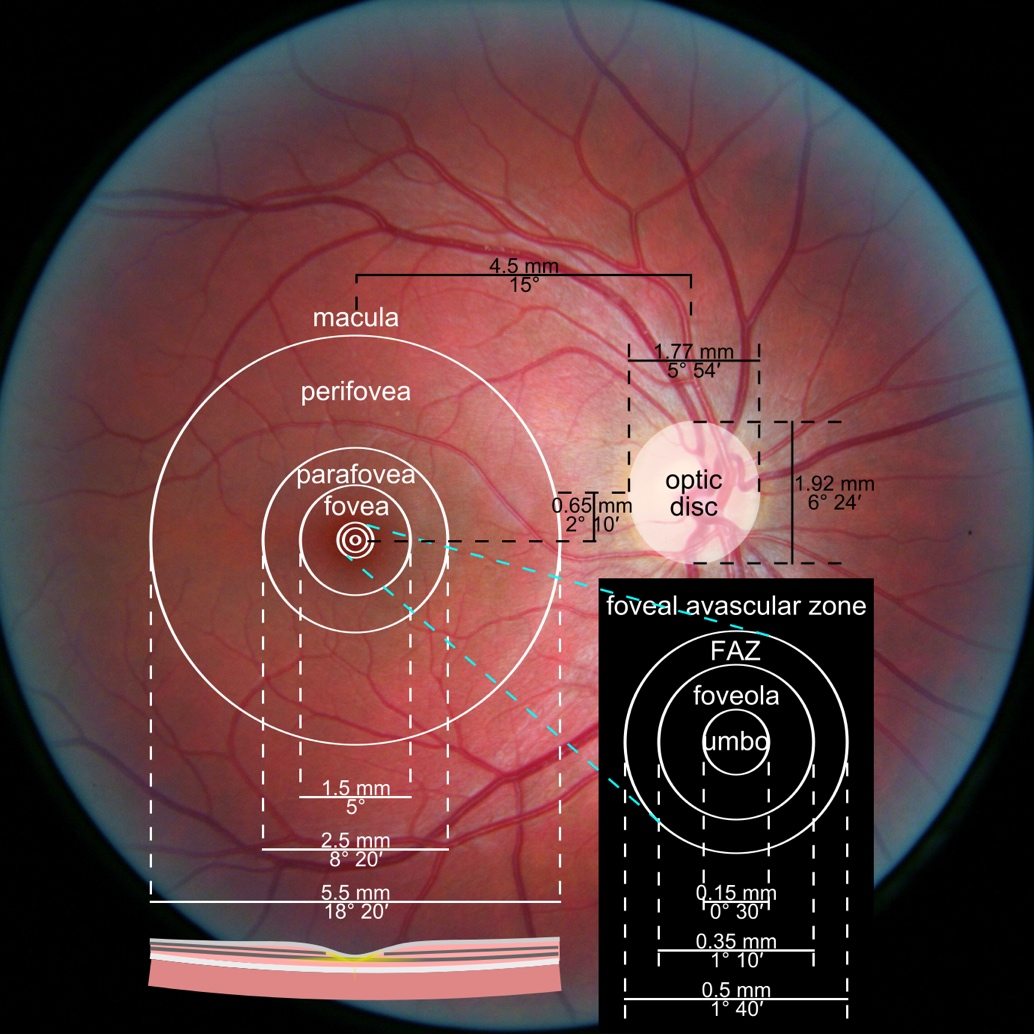
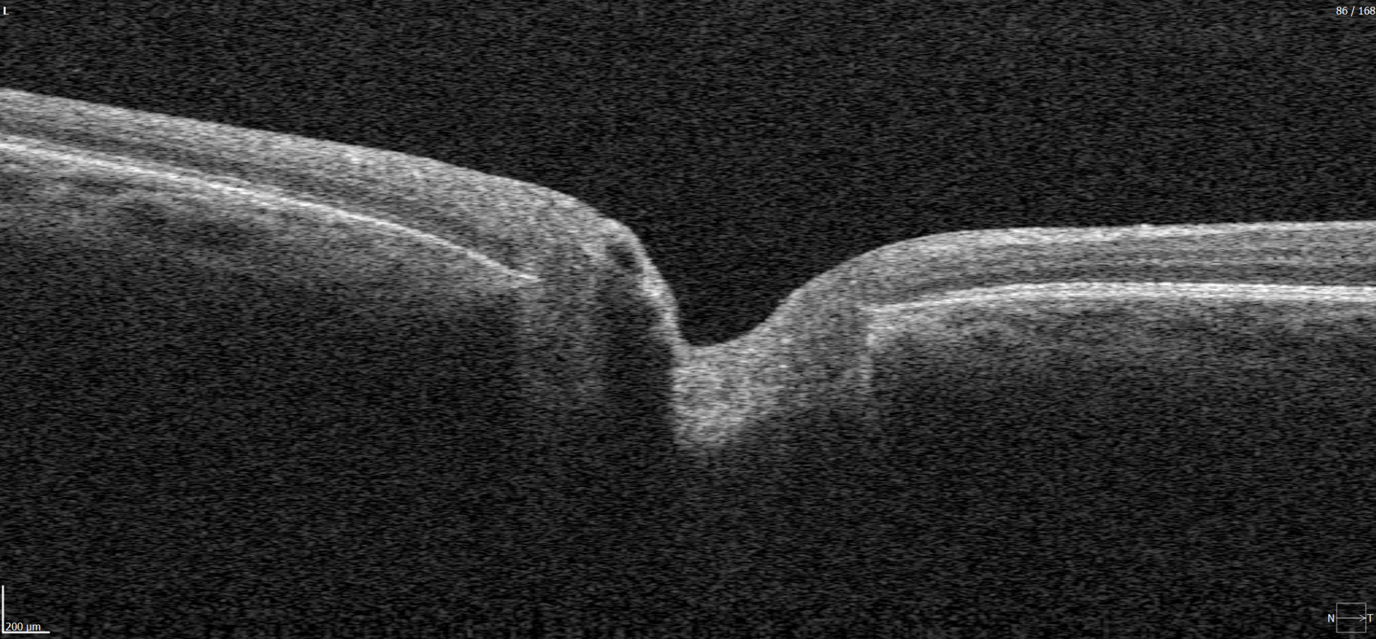
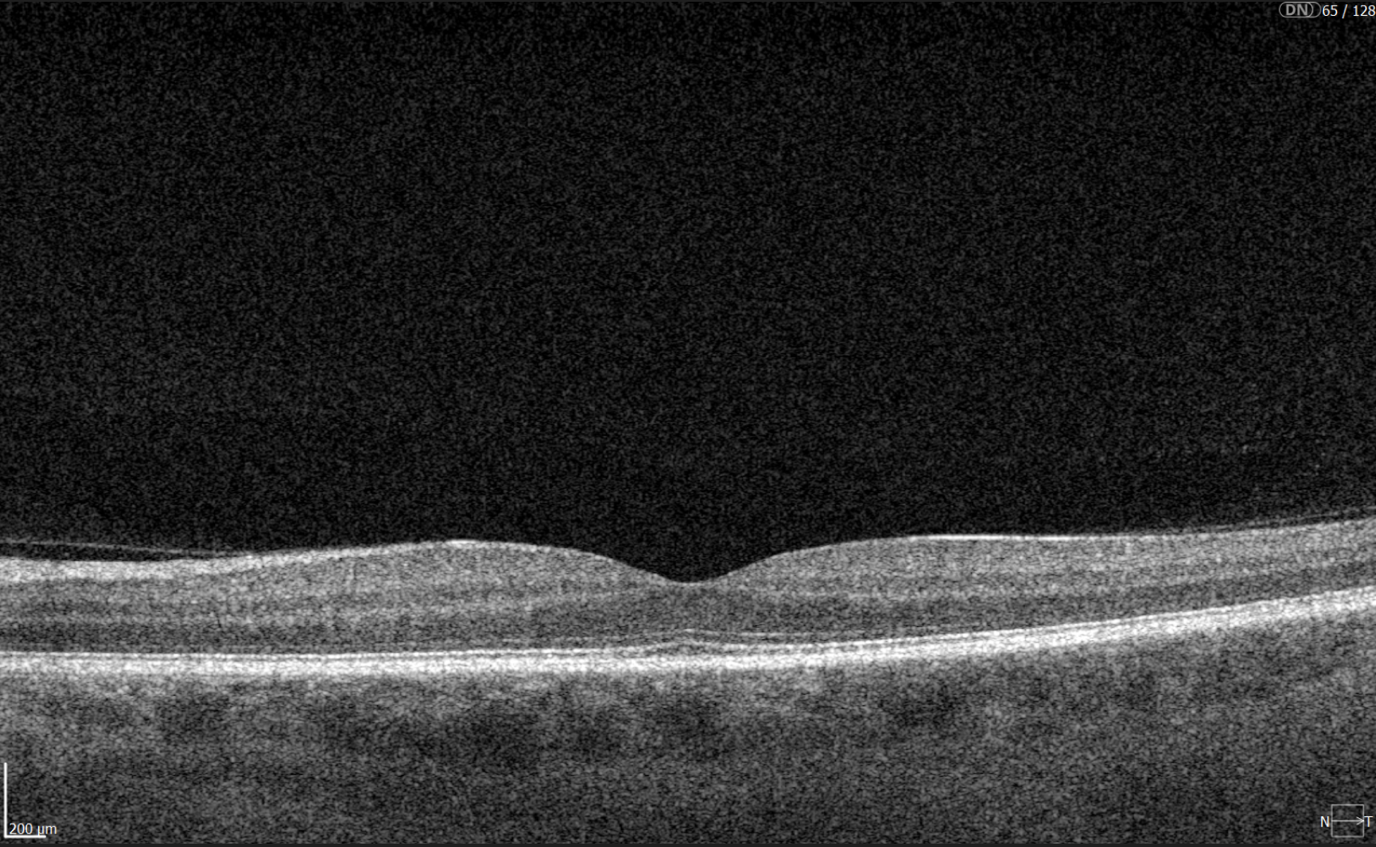
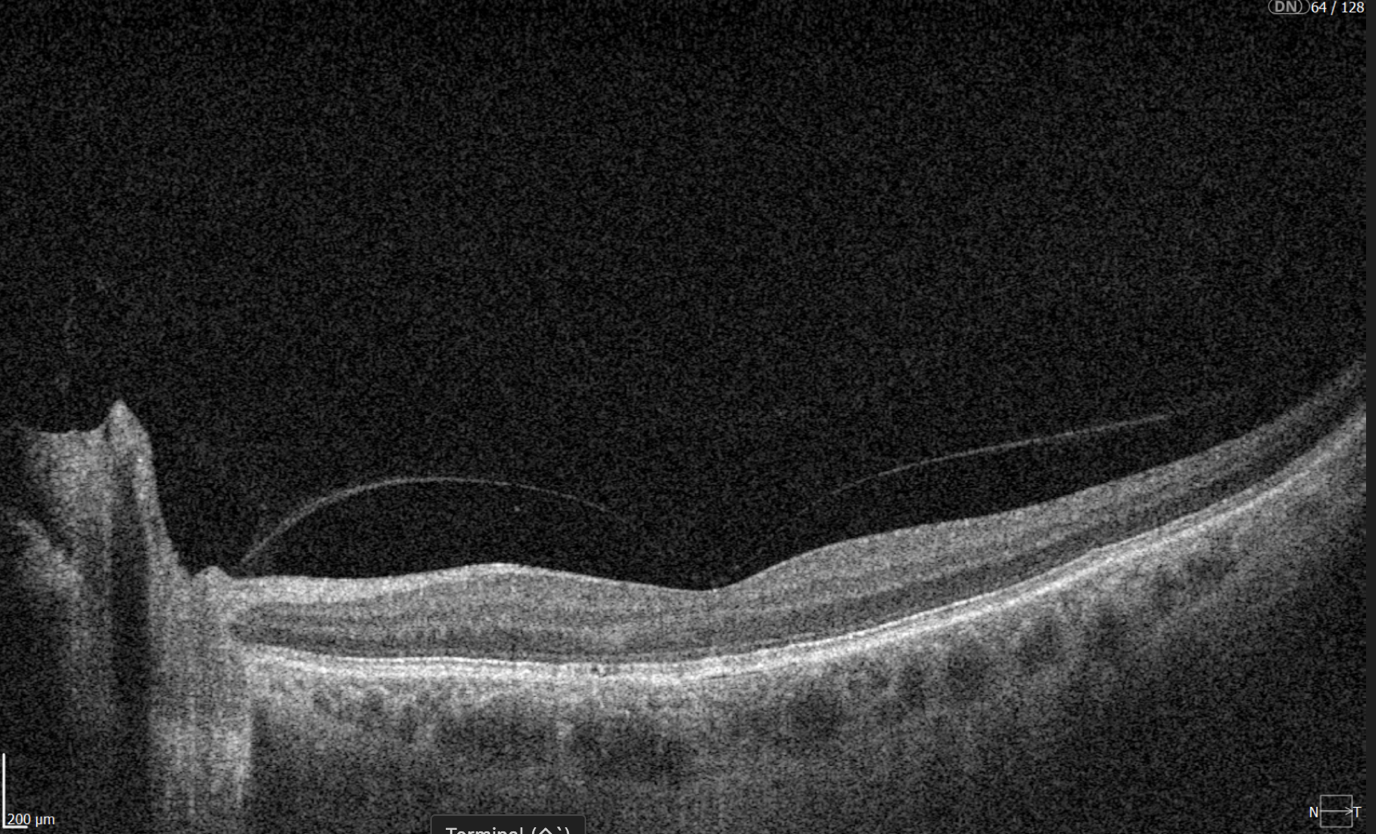
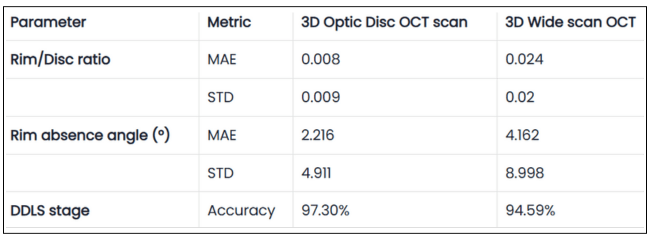
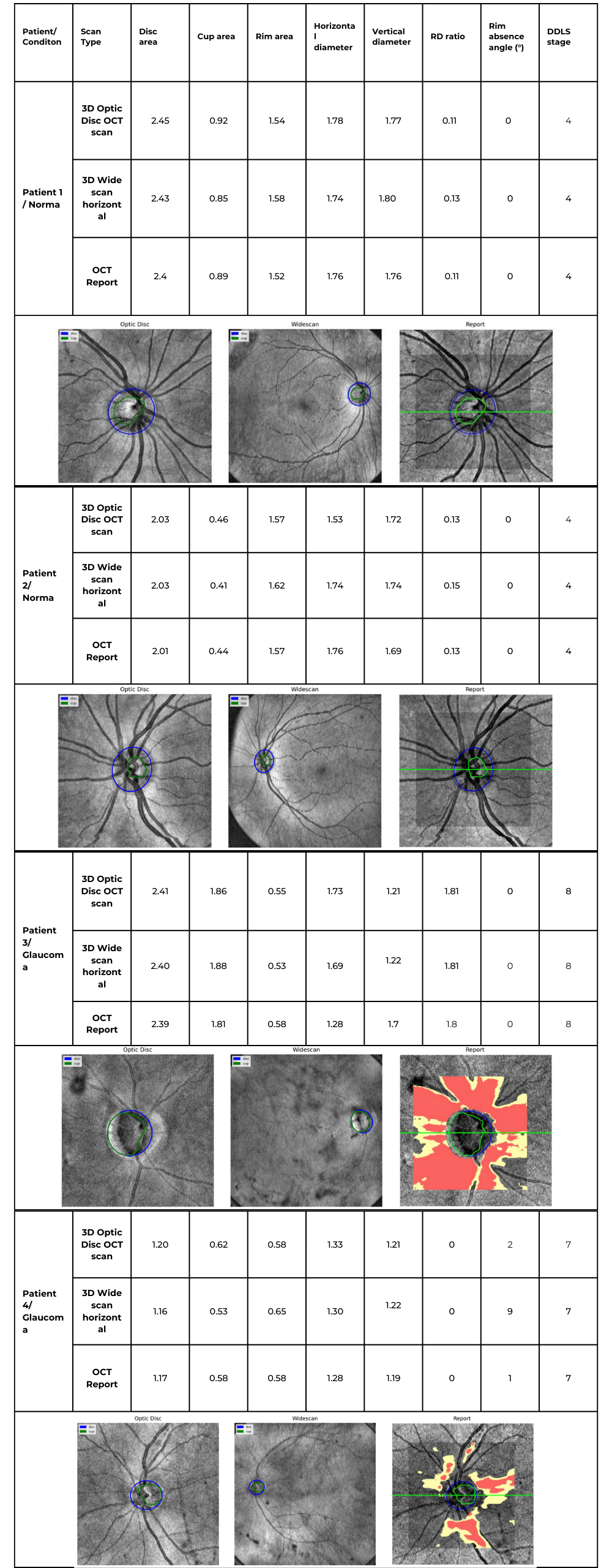
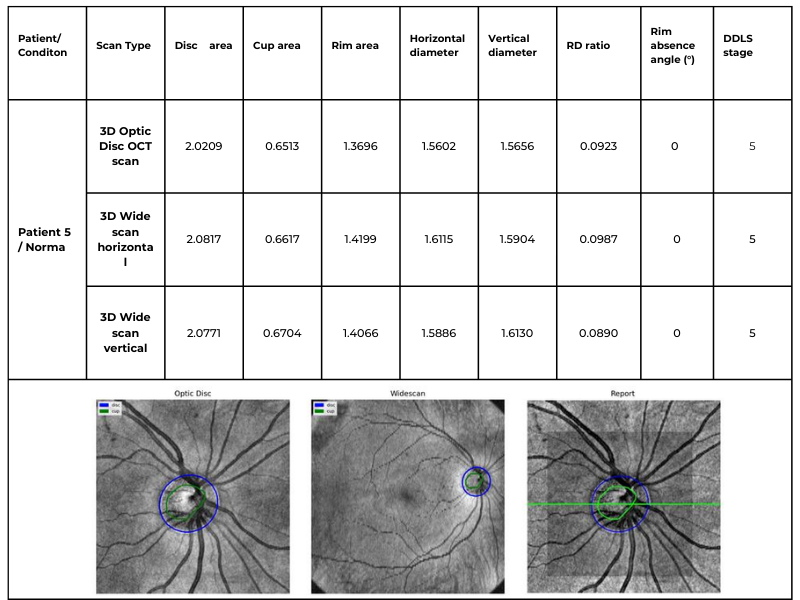
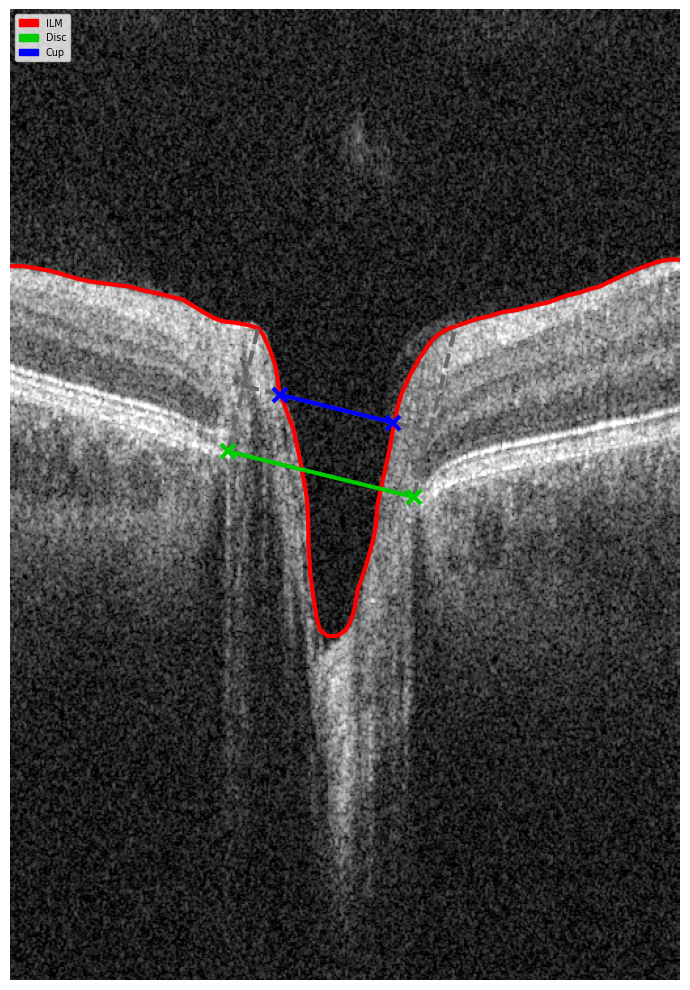
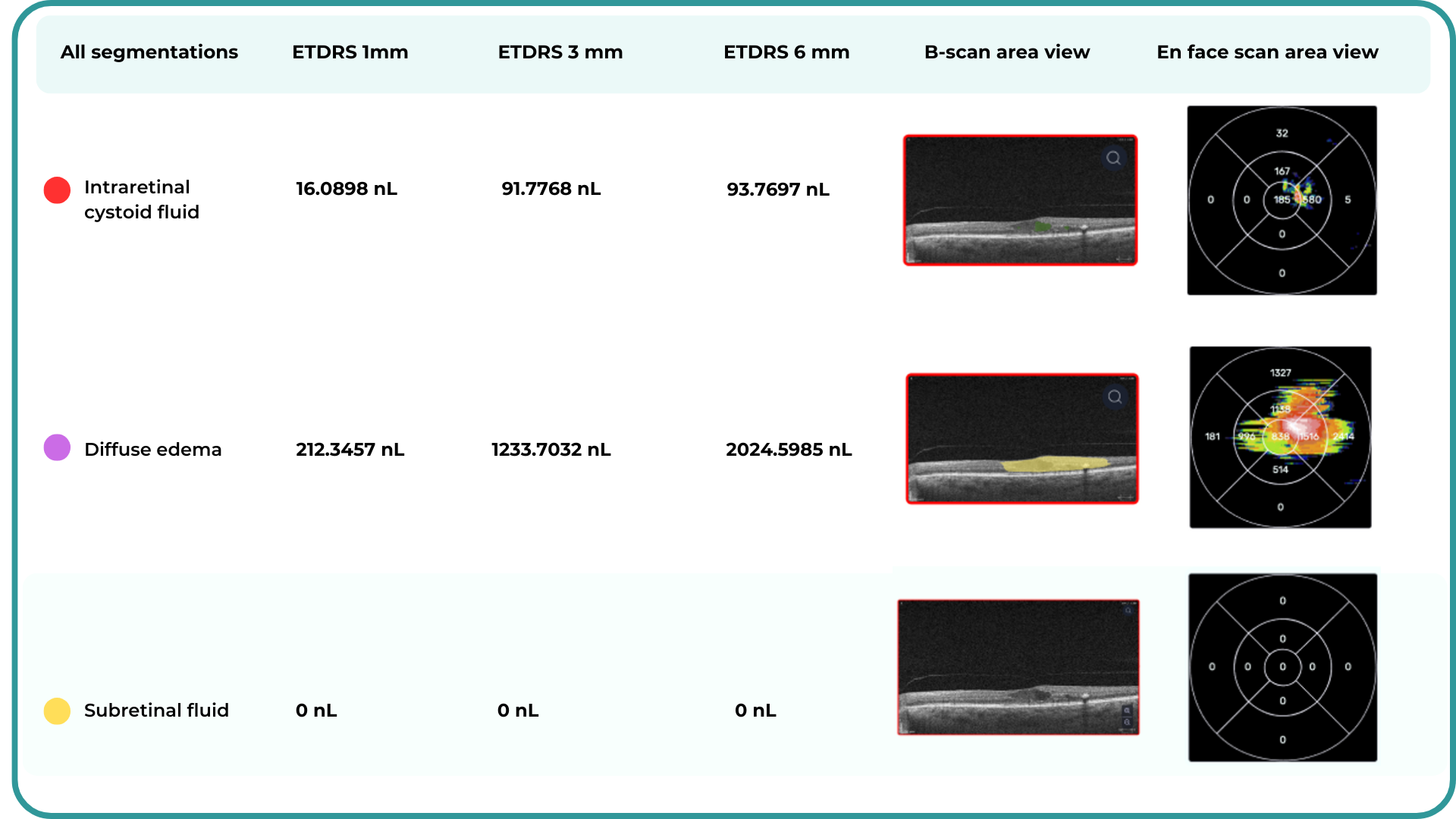
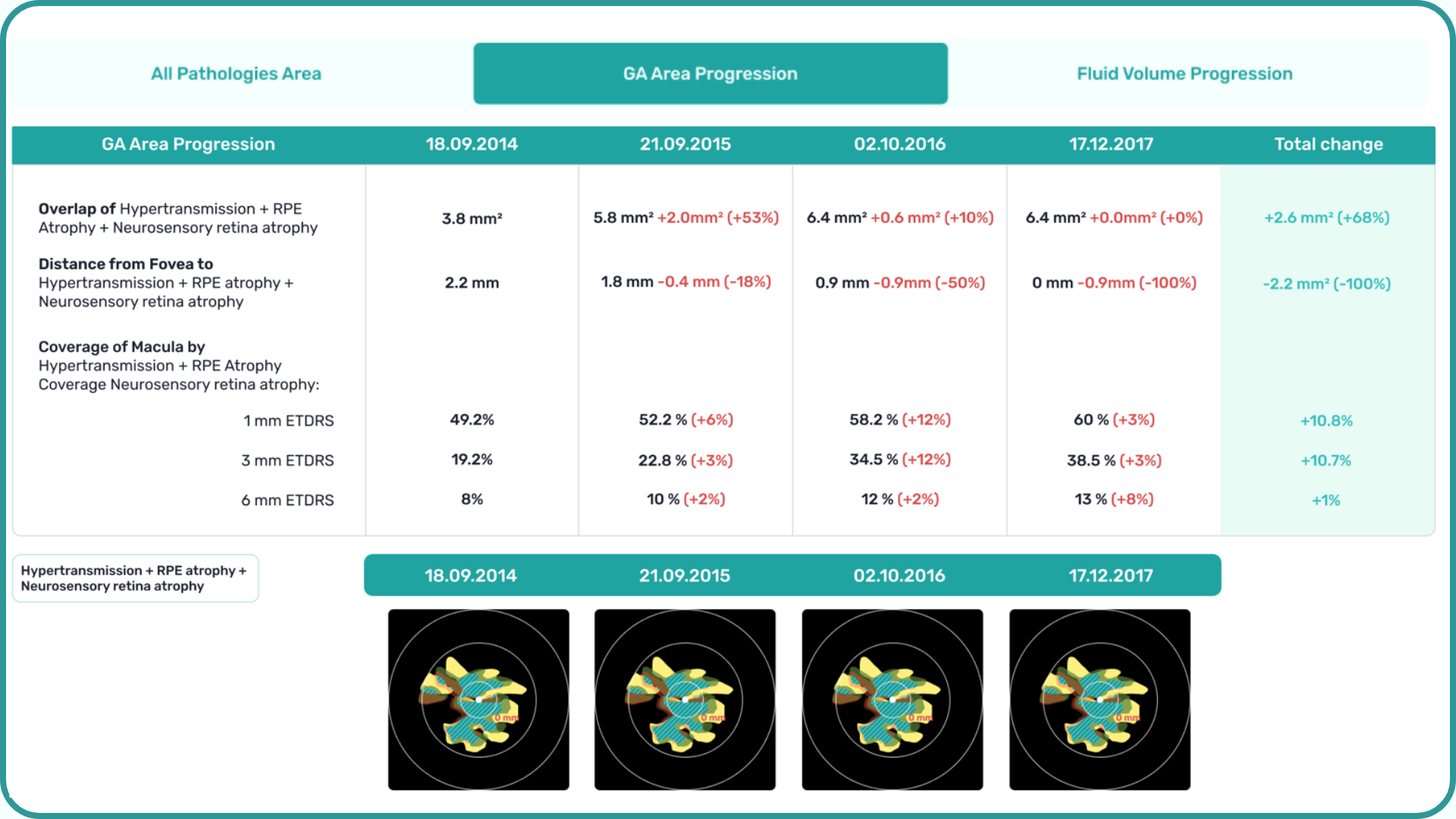
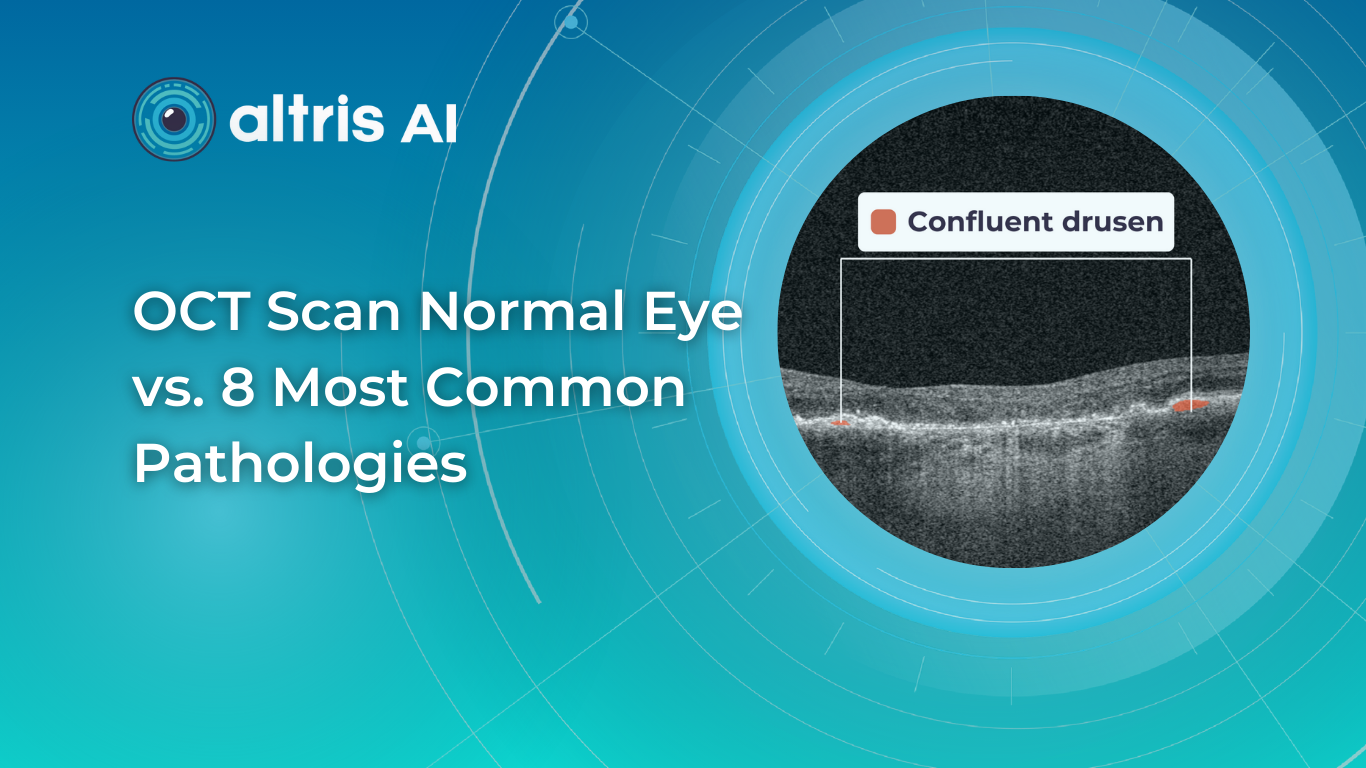
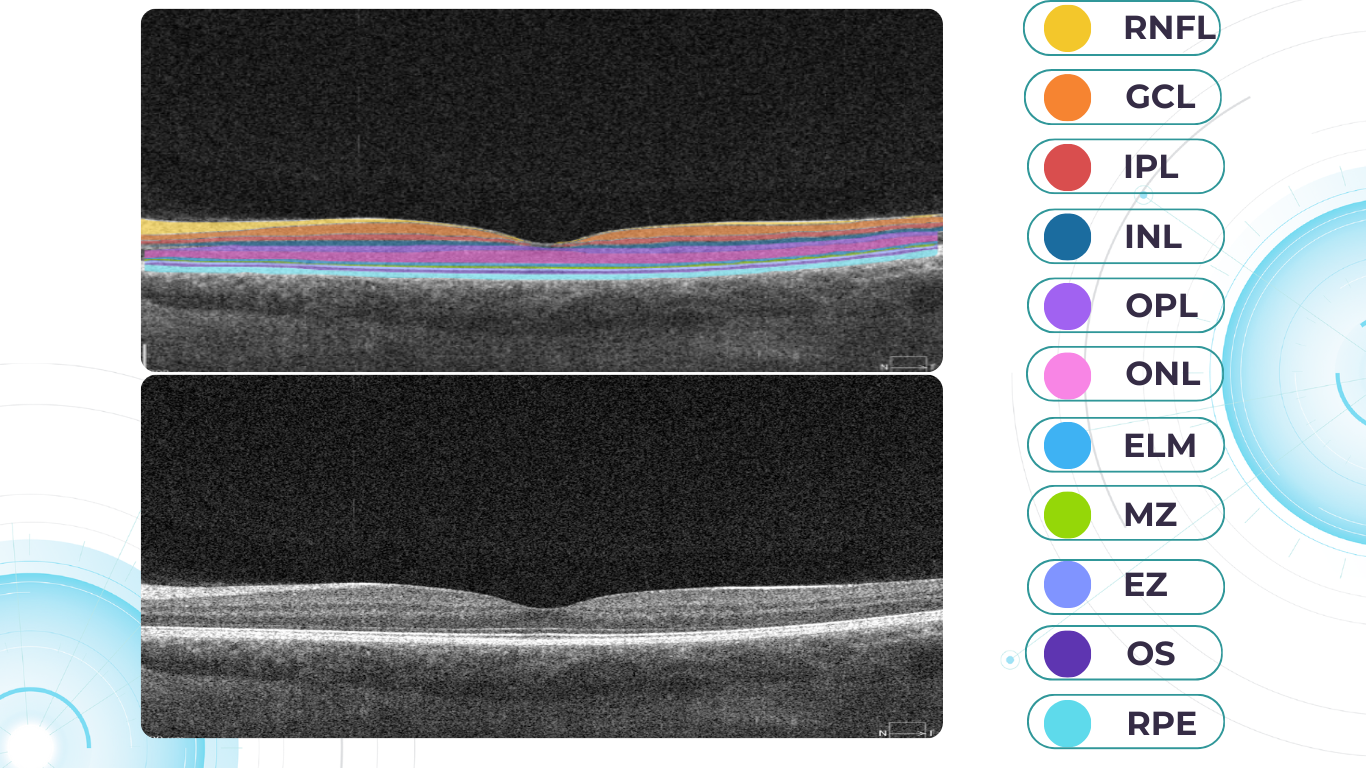
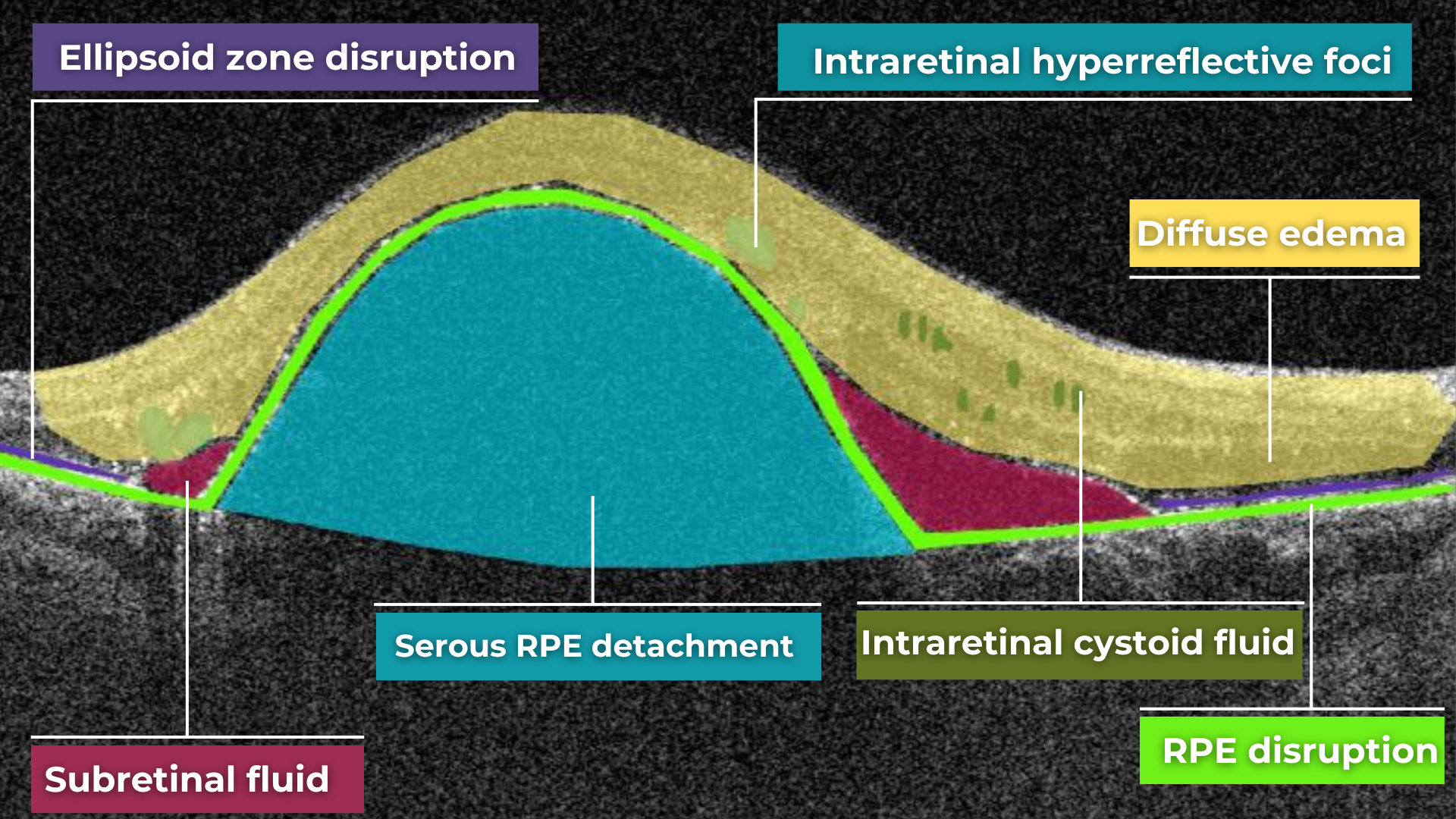
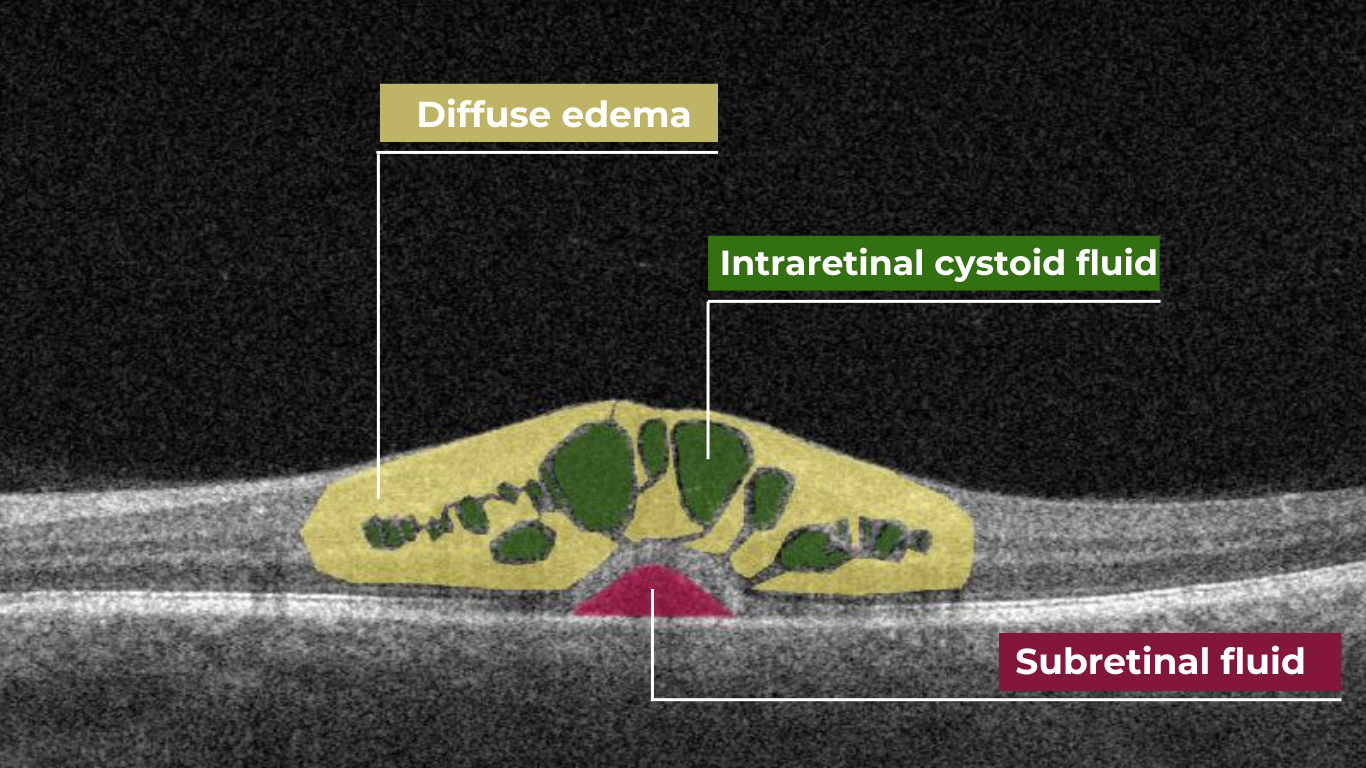
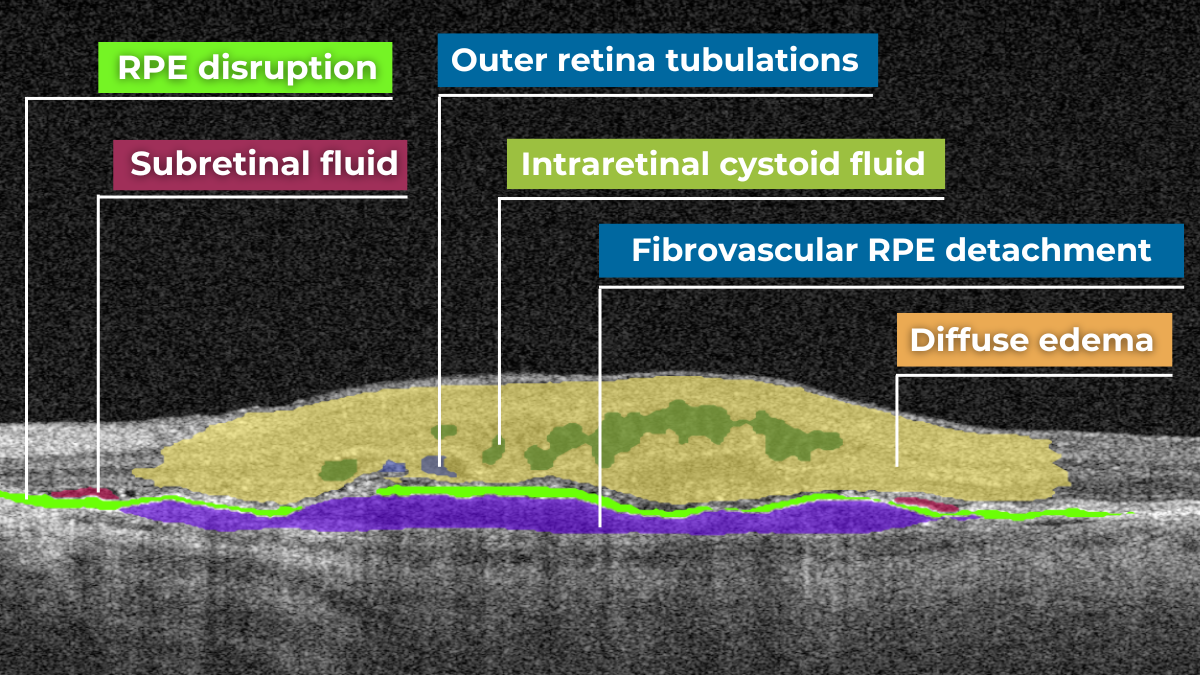
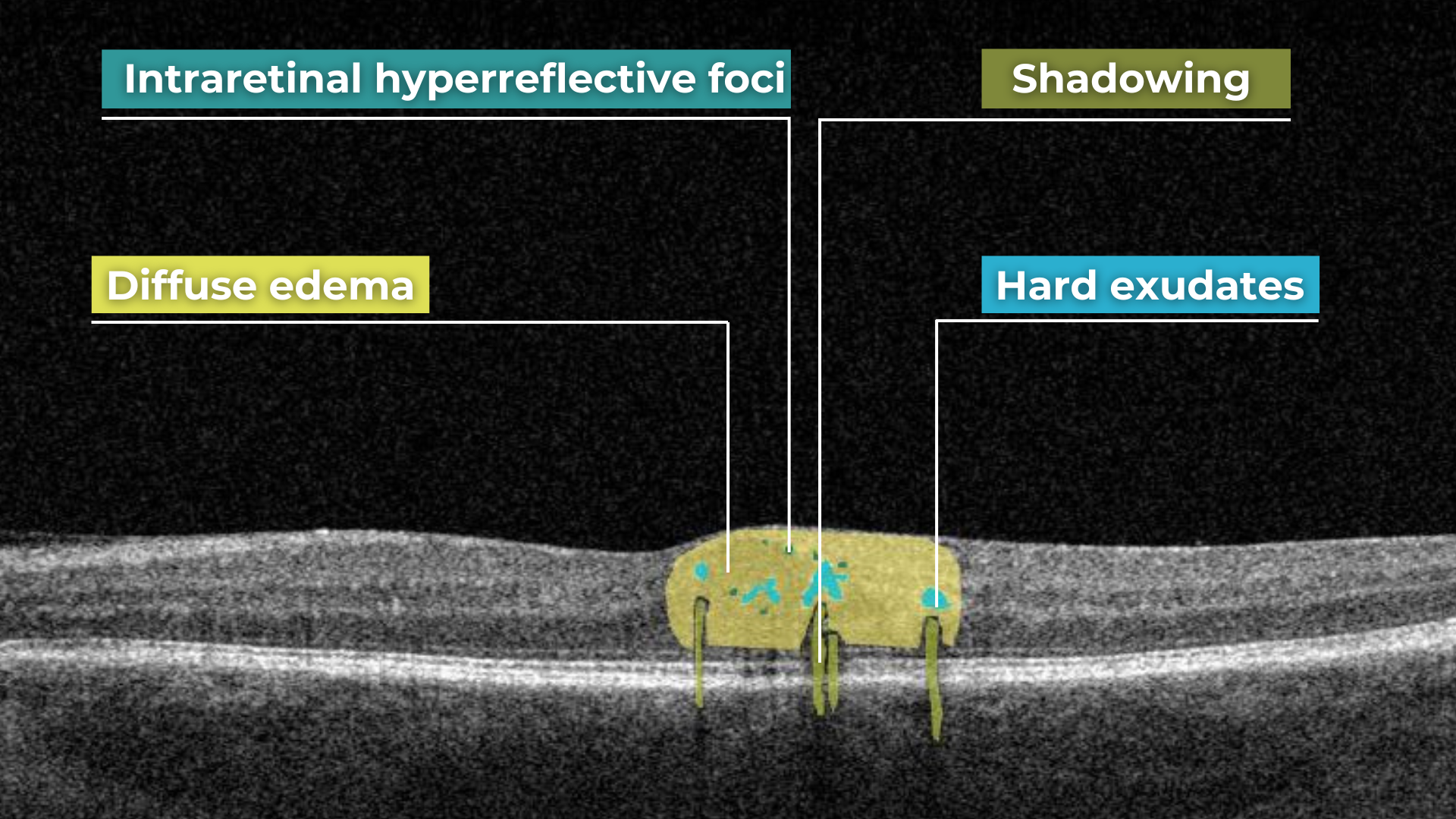
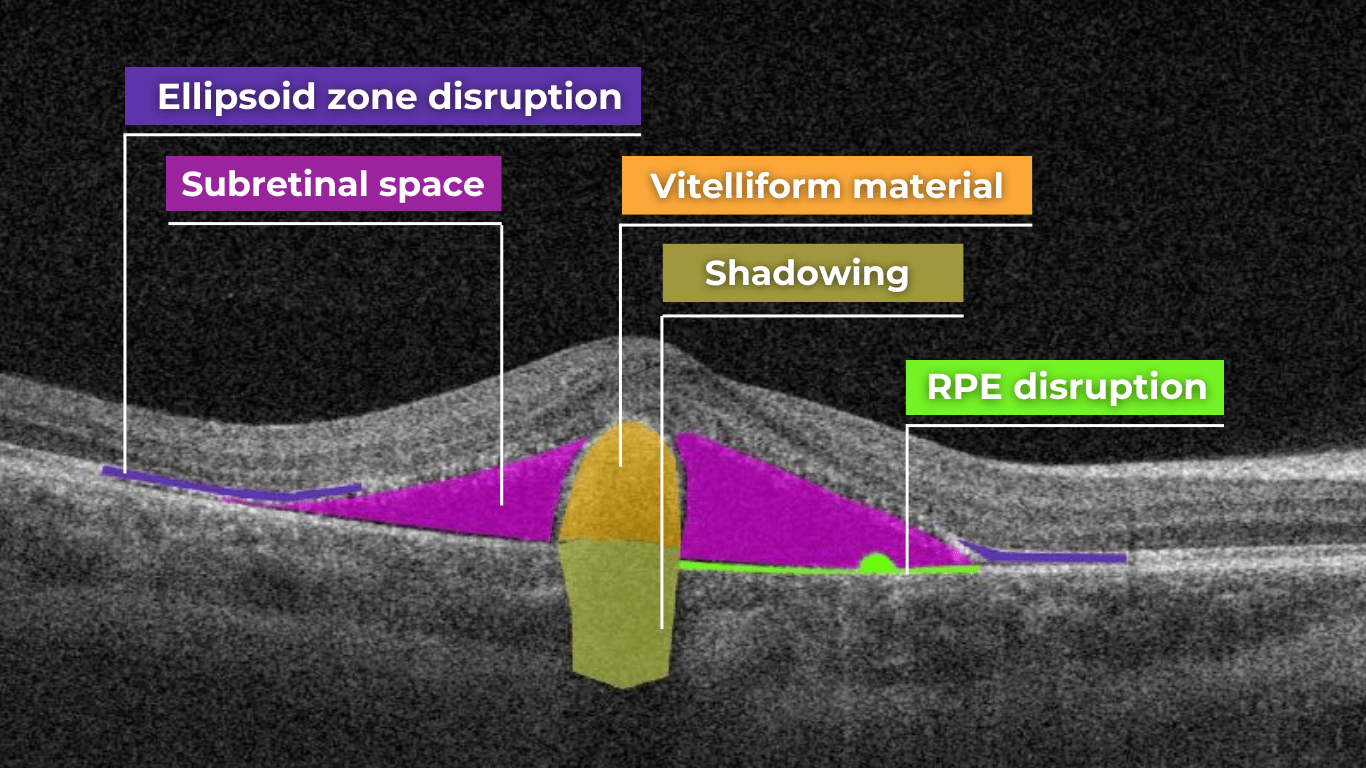
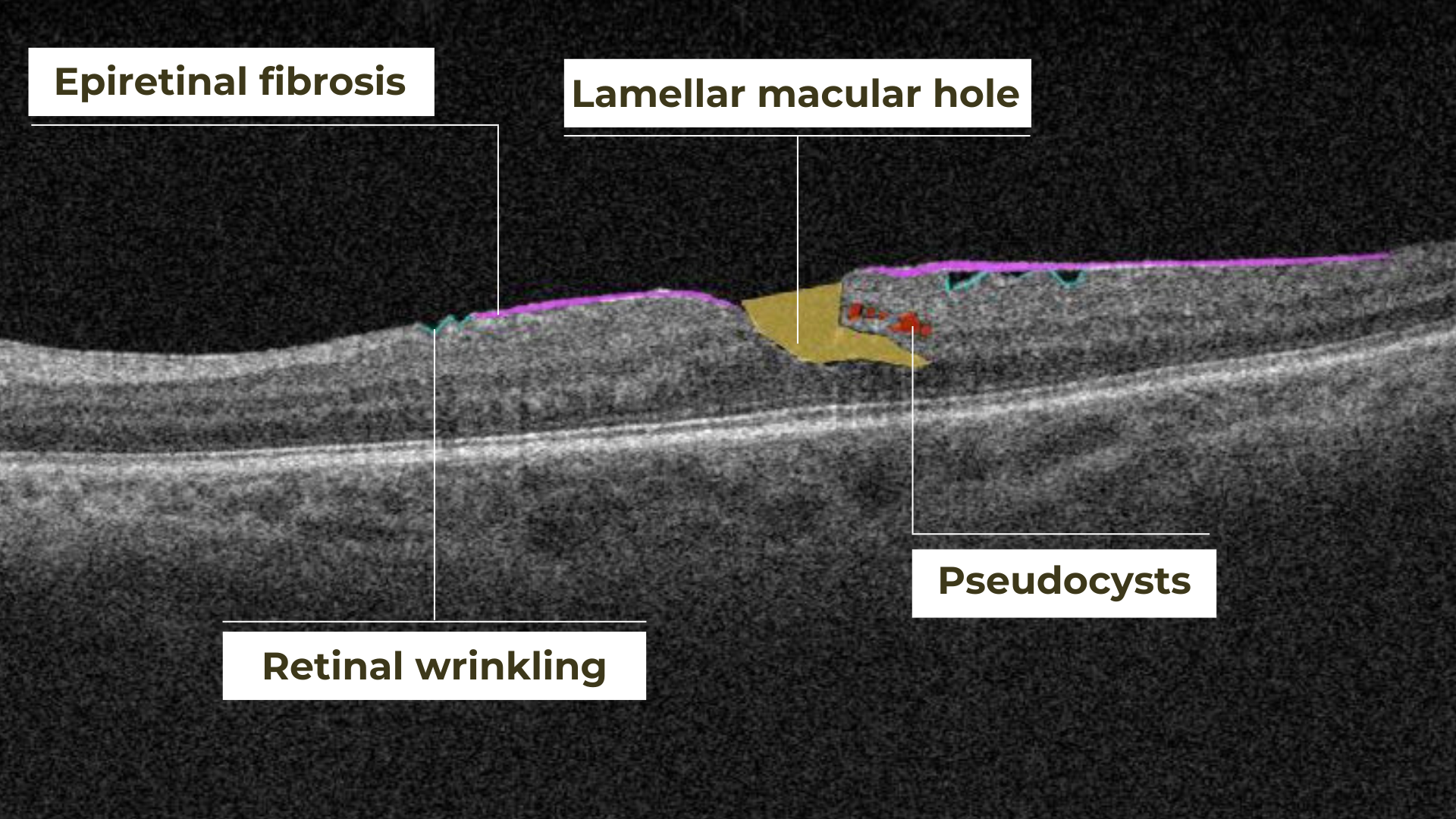
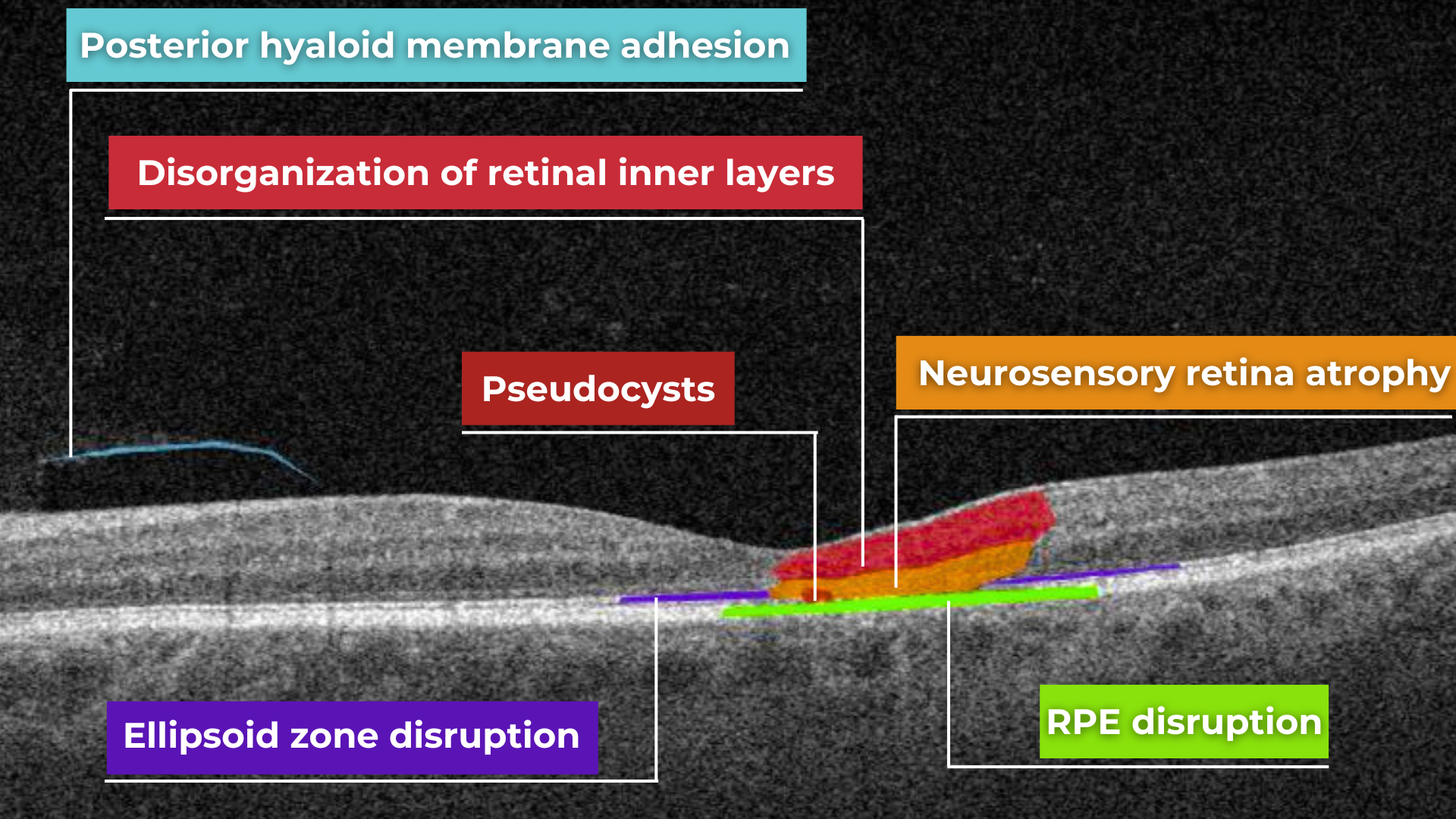
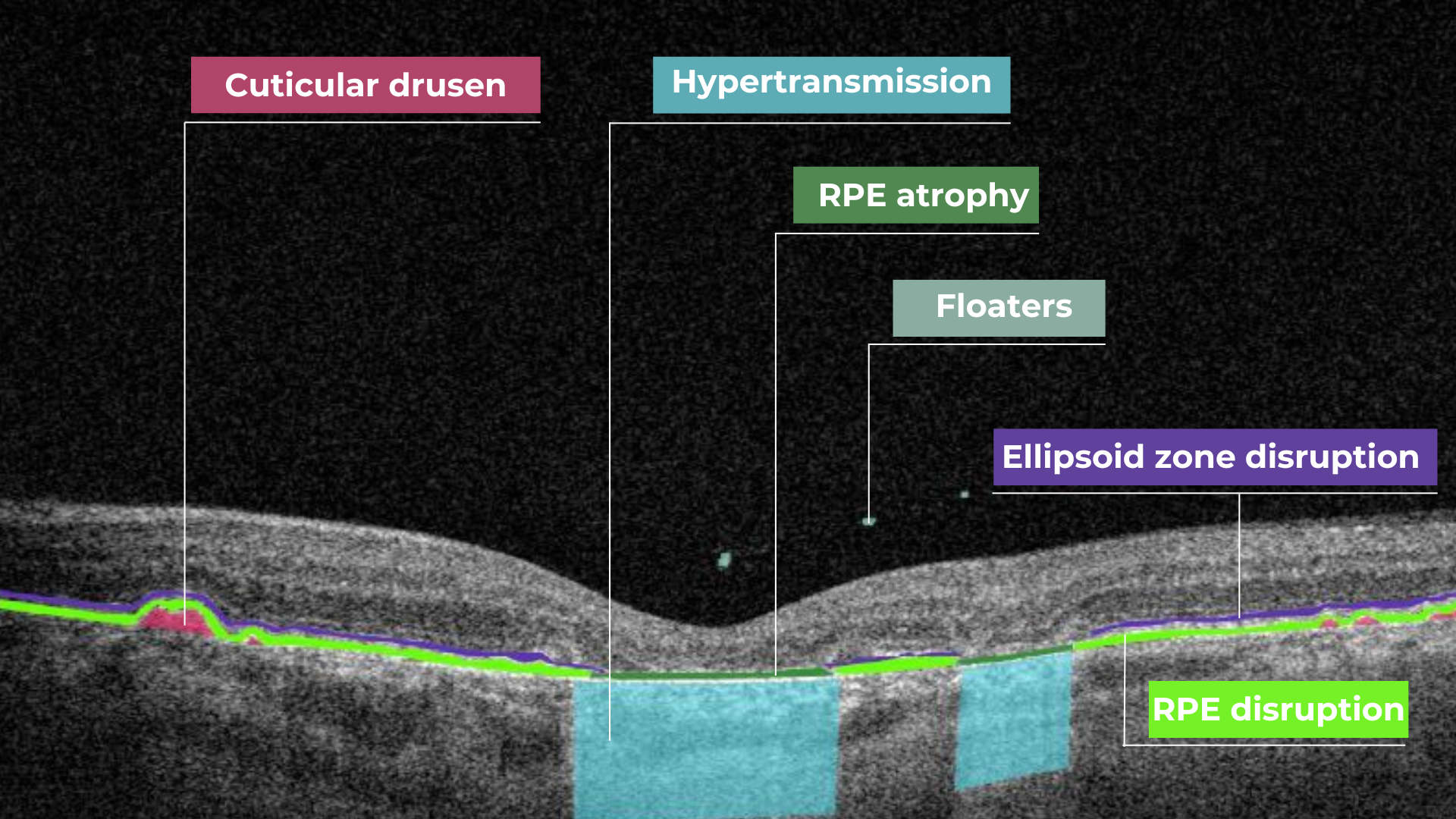 Hypertransmission on OCT
Hypertransmission on OCT