Recently Posted
-

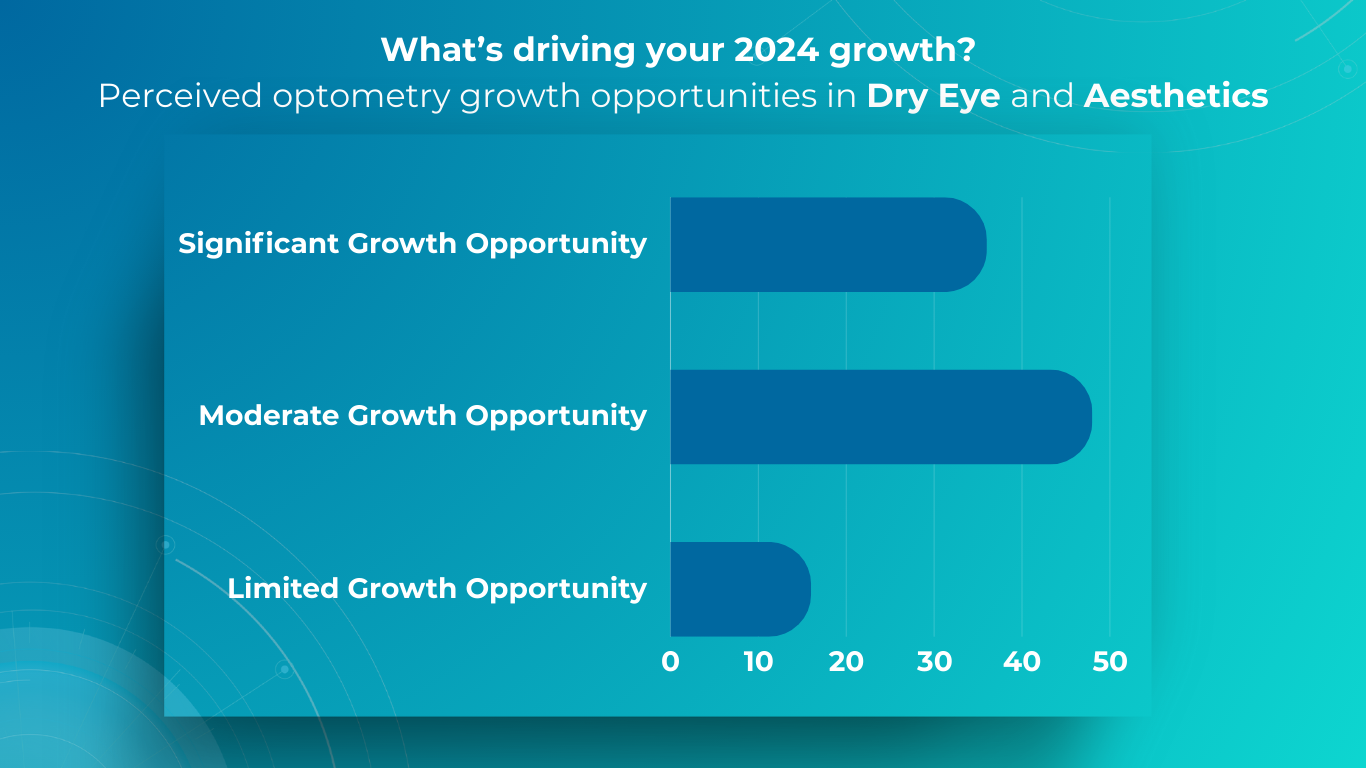
Optometry Practice Growth: Business Cases
 Altris Inc.
03.10.20248 min read
Altris Inc.
03.10.20248 min readOptometry practice growth: business cases
The client. Dr. William C. Fruchtman’s Optometry Practice, owned and operated by Dr. William C. Fruchtman, O.D., is located in East Rutherford, New Jersey, an inner-ring suburb of New York City. With over 30 years of service to the community, the practice provides comprehensive eye care, including regular eye examinations, contact lenses, and glasses prescriptions.
Dr. William Fruchtman’s practice continually seeks opportunities to add value to its services. He is cultivating his expertise in dry eye disease and macular degeneration, implementing advanced technologies, and using another effective strategy to expand his patient base – communicating with patients in their preferred language. Knowing that clear communication is vital to good care, Dr. William C. Fruchtman’s team includes members who speak Spanish and Polish. As such, their website is available in both Polish and Spanish, a valuable asset considering the area’s substantial Spanish-speaking population (up to 20% of the local demographic).
While achieving fluency in every language spoken within your community may not be feasible, consider adapting your website and patient materials to include translations in commonly spoken languages. As Dr. Fruchtman’s experience confirms, even a simple greeting in a patient’s native language can create a bond with patients or, at the very least, prompt a genuine surprised smile.

The problem. To establish expertise in specialized services, Dr. William Fruchtman has been committed to effectively managing dry eye disease and macular degeneration. Not so long ago, the practice implemented Equinox Low-Level Light Therapy (LLLT). This advanced dry eye treatment utilizes LED lights to warm the eyelids gently, promoting meibomian gland function and oil release. With dry eye management addressed, Dr. Fruchtman sought an additional tool to both strengthen his decision-making when managing patients with other pathologies, particularly macular degeneration, and increase his optometry practice growth.
The solution. After researching Altris AI, an Artificial Intelligence platform for OCT scan analysis, Dr. Fruchtman was positive that he wanted to try the platform. Following introductory meetings and a quick onboarding with the Altris team, he started a two-week trial. After personally testing the platform, Dr. Fruchtman decided it was an invaluable addition to his practice.

Integrating Altris AI into the practice has notably enhanced Dr. Fruchtman’s confidence and precision in diagnosing and managing eye care disorders. The practice has also gained a significant competitive advantage, as the platform can routinely perform Glaucoma Risk Analysis on existing OCT scans, offering additional value to patients.
Thanks to the color-coded and labeled OCTs, optometry facilitates patient education and enables practitioners and patients to monitor the progression or treatment results more effectively.
How to grow an optometry practice: more cases from optometry owners
Optometrists undergo years of education, training, practice, and continuous learning – understandably, it is hard to see additional time or resources to pursue business education.
Many practitioners experience stress, balancing patient care demands with the realities of running a profitable business. This feeling can intensify when attending countless conferences and webinars highlighting thousands of ways to make business more efficient. While they offer valuable advice, it’s sometimes helpful to remember simple points of how successful optometry practice growth will look: attracting new patients, retaining existing ones, and ensuring a smooth and efficient workflow. These (even though overly simplified) points allow you to focus on the most critical details.
But before diving into ways of optometry practice growth, remember that the first step is a realistic assessment of your current situation.
While you’re likely aware of some issues, feedback from your team and patients can provide insights, and sometimes even immediate solutions, for areas of improvement.
Even though we cannot directly assist in assessing your specific practice, as you know it best, below we offer some key, proven strategies for growing your business.
Optometry practice growth: expanding your patient base
- Dry Eye Specialization
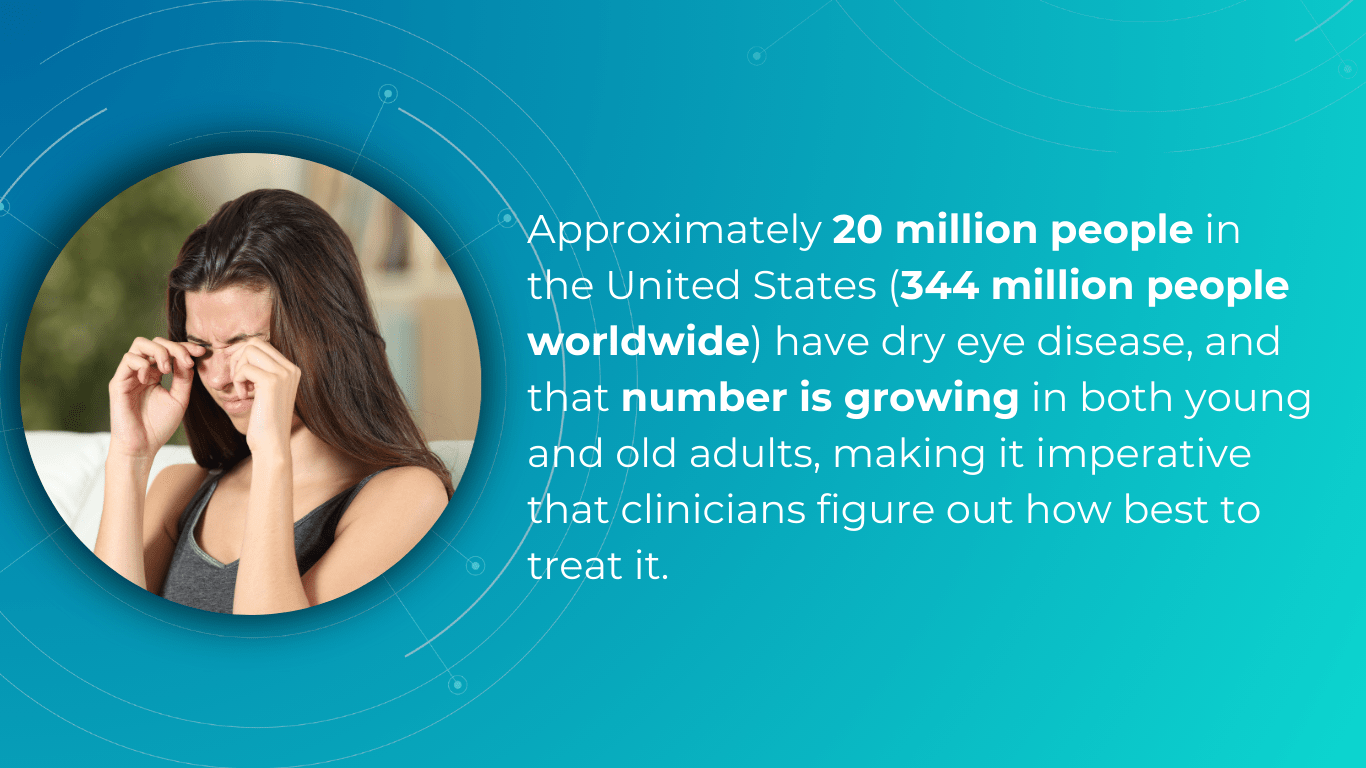
One effective strategy for optometry practice growth is to expand the scope of services to include the diagnosis and management of ocular diseases. For example, dry eye disease (DED) affects ∼344 million people worldwide and over 20 million in the United States alone, yet many remain undiagnosed and untreated. This presents a significant opportunity to care for a large and often underserved patient population. By developing expertise in DED and offering specialized treatments, you can not only attract new patients but also contribute to improving the quality of life for those suffering from this chronic condition.

There are numerous approaches to managing DED effectively. As mentioned, Dr. William C. Fruchtman’s practice utilizes Equinox Low-Level Light Therapy (LLLT).
Dr. Shane Swatts, O.D., owner of Eastern Virginia Eye Associates, employs AI software to enhance DED diagnostics, conduct more comprehensive analyses, and keep detailed patient medical histories. This technology upgrades pre-and post-operative care, saving time without compromising accuracy.

- Aesthetic Optometry
Dr. Janelle Davison identified an opportunity for optometry practice growth by addressing patient needs while generating additional revenue by incorporating aesthetic optometry services into her practice. Within a single quarter, her practice generated $14,000 in revenue from aesthetic product sales alone.

Dr. Davison also collaborates with a licensed aesthetician who operates within the practice on a contract basis, sharing the revenue generated from aesthetic services.

- Glaucoma Management
Dr. James Deom, O.D., M.P.H., an optometrist from Pennsylvania, implemented a successful strategy for optometry practice growth based on attracting glaucoma patients, significantly increasing glaucoma-related revenue. He initiated internal marketing efforts by inquiring about patients’ family history of glaucoma and informing them about the practice’s newest technology for the early detection of vision loss.

Practices specializing in glaucoma management can significantly benefit from incorporating advanced software solutions to complement their existing diagnostic hardware. For instance, integrating Altris AI, AI for OCT, into their OCT analysis workflow enables not only automated screening of 70+ pathologies and biomarkers but includes assessing retinal nerve fiber layer (RNFL) asymmetry for glaucoma risk evaluation.
- Patient-Centered Care
Offering diverse channels for patient interaction can broaden your practice’s reach and improve the patient experience. Dr. Melissa Richard, O.D., sought to provide patients with a preview of frame options before their appointments. To achieve this, she integrated Optify technology into her practice, a solution she discovered during a Vision Source Exchange lecture. This technology creates a virtual showroom where patients can explore and select their preferred frames in advance, streamlining the in-office experience.

Patient education is also key to patient-centered care and personalization, which not only empowers individuals and improves their outcomes but also fosters optometry practice growth. Those who understand their eye health are more likely to adhere to recommendations.
A study demonstrates that 94% desire educational content, but a third don’t receive it.
Providing color-coded OCT reports with pathologies, biomarkers, and pathology progression tracking not only satisfies this need but also elevates your practice above competitors.

Improve efficiency in the optometry office through strategic partnerships & team building
When optometrists consider further career development, they may seek additional support to achieve their goals. Dr. Linda Enciso, O.D., found such support when her practice joined the AEG Vision family in 2019. The transition brought numerous positive changes, boosting patient care and fostering growth opportunities for team members.
Although Dr. Enciso had already been operating her practice for 13 years and had implemented electronic health records (EHR) systems and third-party software to improve patient communication and boost optometry practice growth, her goal was to continue these advancements and expand the scope of practice. Joining AEG Vision allowed her to transition to the training team, access continuing education opportunities to stay informed about advancements in optometry and healthcare, collaborate with other healthcare providers and cross-functional teams to enhance comprehensive patient care.

While the phrase “team building” might evoke images of complicated activities and extensive effort, fostering a strong team can be achieved through simple, engaging initiatives. Consider the inspiring example of Dr. Jonathan Cargo, O.D.
Dr. Cargo recognizes the value of personal development through reading but finds it challenging to share his insights with his team effectively. Inspired by his wife’s long-standing book club, he initiated an office book club to encourage team connection and shared learning to improve efficiency in the optometry office.
The book club operates with team members suggesting relevant titles and collectively reading chapters over a month, dedicating time during team meetings for discussions. Dr. Cargo highlights the recent success of reading “Crucial Conversations,” a selection prompted by team members’ desire to deepen their communication skills, particularly in navigating challenging discussions with colleagues, patients, and even family members. The shared reading experience gave a better understanding of effective communication strategies and empowered the team to navigate difficult conversations.

Summing up
When regarding optometry practice growth, consider the time, effort, and resources you are prepared to invest. To expand your patient base, explore the addition of new services.
To optimize costs and efficiency and gain a competitive edge, investigate the possibility of implementing AI in your practice – it can be a second-opinion tool, or you can read here how practitioners use it for marketing, creating educational materials, and more. To encourage staff retention and nurture a positive work environment, prioritize team-building activities; even seemingly simple initiatives can produce significant benefits.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

Optometry Trends in Action: 12 Real-World Success Stories
 Maria Znamenska
17.09.20248 min read
Maria Znamenska
17.09.20248 min readOptometry Trends in Action: 12 Real-World Success Stories
Optometry trends explained: showcasing real-world optometry practice owners who are adapting to the shift in patient needs, successfully implementing solutions to automate routine and laborious tasks, using AI to combat staff shortages, creating their own brand mascots, and more.
Optometry trends for the patient journey: digital communication
Online shopping, global deliveries, and instant brand replies through messengers have dramatically shifted client expectations and behaviors. The ‘convenience economy’ isn’t slowing down, pushing businesses to adopt technology for more streamlined consumer experiences.
What does this mean for your practice? Your patients now expect fast and efficient communication across all touchpoints – from online scheduling to contactless payments. Transforming your practice to meet these demands ensures satisfied patients and contributes to long-term success, as any optometry practice thrives on the individual experiences of the patients it provides.
46% of optometrists reported that patient expectations have risen since the pandemic.
Practices can optimize their workflows in various ways, but generally, the goal is to automate routine administrative tasks, free up staff, and reduce patient waiting time. Digital safety forms and document management systems eliminate physical paperwork, while online proofing and approval systems speed up document processing.

Dr. Justin Bazan, owner and optometrist at Park Slope Eye, New York, has taken this even further by eliminating phone calls at his office entirely and is pleased with the results. This solution was based on several months of analyzing data related to phone calls, including time spent on calls and the frequency of missed calls. The team recognized that while the staff could simultaneously chat with multiple patients, they could only handle one phone call at a time.

Chad Fleming, OD, Owner and OD at Wichita Optometry, Kansas, also identified the need for an enhanced digital presence to prioritize patient convenience. His practice faced the challenge of managing a high volume of phone calls and text messages, requiring either additional staff hiring without an immediate increase in revenue or a strategic reallocation of existing personnel.

Dr. Fleming optimized the patient experience by setting up automated checkouts at some of his practice locations. This approach enabled him to reassign three front desk employees to the digital communications team. While the transition required patient education to familiarize them with the virtual check-in process on iPads, it did not result in patient attrition.
Brianna Rhue, OD, Owner and Optometrist of West Broward Eyecare Associates, Florida, agrees that the traditional approach of answering calls and checking emails once a day differs from today’s patient expectations. She advocates step-by-step optimizations throughout the patient journey to eliminate unnecessary wait times and increase productivity.

Upgrading to a more advanced EHR system is one of the significant opportunities to streamline practice operations, save practitioners time, money, and stress, and align with optometry industry trends. Unfortunately, once hailed as revolutionary, some widely adopted EHR solutions are now criticized for their burdensome workflows and counterintuitive interfaces. This has led some practitioners to describe their interaction with systems as “death by a thousand clicks.”
By leveraging up-to-date EHR features like customizable patient encounter templates, integrated imaging and diagnostic tools, and patient outcome tracking, eye care professionals can shift their focus from paperwork to patient care.
Another of optometry trends gaining momentum among optometry practice owners is offering flexible payment options. This reflects not only the growing demand for convenience but also the financial constraints of patients navigating the current economy that is heading to a recession.
Dr. Rhue encourages practices to adopt mobile payment solutions that enable patients to pay electronically using platforms like Apple Pay, Venmo, or PayPal at the point of service. For balances due after the visit, the ability to send secure payment links via text message can greatly enhance the collection process.
Furthermore, providing patient financing options empowers patients to choose how and when they pay. This offers additional convenience for both parties and eliminates friction by allowing patients to spread the cost of their care over time rather than requiring full payment upfront.
If you are still determining which technologies of these optometry industry trends your patients will be eager to adopt, consider the approach taken by Scott Jens, OD, the owner of Isthmus Eye Care, Wisconsin. Dr. Jens has successfully implemented post-examination surveys to gather patient feedback. This strategy serves a dual purpose: demonstrating your commitment to patient satisfaction and gaining valuable insights into which technological advancements would most benefit your practice.

Optometry trends in the exam room: tech-driven precision and patient education
Optometry relies heavily on technology, and investing in hardware upgrades is a significant financial commitment. However, if your hardware needs are met, but you still want to be at the forefront of technological advancements, consider specialized software and platforms to extend the possibilities of your existing devices.
Dr. Maria Sampalis, OD, the owner of Sampalis Eye Care, Rhode Island, utilizes two such programs in her practice. To support her specialization in dry eye management, she employs CSI Dry Eye. Additionally, she uses Altris AI, an AI-powered platform for OCT scan analysis, to provide a second opinion and enhance diagnostic accuracy.
Dr. Sampalis finds that the Dry Eye software allows her and her staff to analyze symptoms and images comprehensively, improving patient care, time savings, and increasing diagnostic precision. See how OCT AI works here.
Her patients also appreciate Altris AI, which analyzes OCT scans for over 70 pathologies and biomarkers while also calculating the risk of developing glaucoma.

Working with specialized software solutions improves diagnostic accuracy and aids in patient education. Visual representations of their conditions, facilitated by these technologies, empower patients with a clearer understanding, leading to increased treatment compliance.

Eye Place, an optometry center in Columbia, also leverages Altris AI, among other cutting-edge technologies. They capture images using the Topcon Maestro2 OCT and use Image Net6 software to export DICOM files to the Altris AI platform.

Beyond AI-powered OCT analysis, Eye Place utilizes state-of-the-art diagnostic tools, such as 3D OCT equipment, to screen for serious conditions, including glaucoma, diabetes, and macular degeneration. Furthermore, they work with AdaptDX Pro, a technology capable of detecting macular degeneration earlier than traditional methods.
Another case of optimizing and enhancing the exam process is West Broward Eyecare Associates. They implemented Optify, a smart building solution offering full fiber connectivity. Patients can pre-select frames in the online optical store before their visit, streamlining the in-office experience. Additionally, the practice utilizes Dr. Contact Lens, a platform for convenient ordering, reordering, and prescription management for contact lens wearers, reducing paper waste.
There are also advancements in AI transcription technology that are poised to ease clinical documentation and automate a traditionally laborious task.
The adoption of AI in clinical documentation has been shown to reduce the time doctors spend on charting by approximately 2 hours per day.
AI exam transcription is still in the process, and the existing possibilities are not yet flawless—struggling with patient responses like “mm-hm” and “uh-huh”—the technology is evolving, promising greater efficiency and accuracy in the future. For example, one such program starts the transcription process of the exam by confirming patient consent and a click of the record button by the optometrist. Then, AI captures, structures, and summarizes information in real-time, filtering for relevant details to generate documentation for each patient appointment.
Optometry trends for competitive advantage: using AI in Marketing and Decision-making
Some practice owners may still believe their patient demographics do not necessitate an expanded online presence, particularly when considering elders. But you should be different from your competitors.
The reality is that today’s patients, regardless of age, are increasingly turning to the Internet for information and services. While word-of-mouth referrals remain valuable, a solid online presence is essential for practice growth and visibility in today’s competitive landscape.
Twin Forks Optometry and Vision Therapy in New York reports that their most effective marketing strategy involves a monthly-to-quarterly newsletter distributed to existing patients. This newsletter highlights practice updates, recent vision therapy graduates, new podcast episodes, and seasonal information. They’ve also observed that educational posts generate significant engagement and have even led to new patient visits.

Voice Search Optimization (VSO) is emerging as one of the new trends in optometry that has the potential to benefit practices significantly. Dr. Brianna Rhue, OD, co-owner of West Broward Eyecare Associates in Florida, asserts that a search engine optimized (SEO) website alone will soon be insufficient for patients to discover your practice online easily, especially in highly competitive locations.

Contrary to popular belief, it’s not just the tech-savvy individuals who rely on voice assistants. This technology is predominantly used by older individuals who haven’t mastered typing or face difficulties with it.
However, while the benefits of digital communication are undeniable, it’s crucial to acknowledge that it often adds up yet another layer of responsibility to already overburdened teams. This is why generative AI tools like ChatGPT and Gemini are gaining popularity among optometrists, offering solutions to this and other challenges.
For example, Dr. Ryan Cazares, the owner and founder of Scott Eye Care in Louisiana, utilizes ChatGPT to generate social media and educational content for his practice. He brainstorms with AI content ideas, creates visuals for social media and marketing campaigns, and has even developed a unique mascot (Dr. Seymour) that engages his audience.

The practitioner also uses AI to generate personalized educational materials for their patients. Traditionally, his practice relied on generic Optometric Association pamphlets, but now, it has transitioned to simple one-page educational sheets tailored to individual patient needs.

Dr. Haley Perry, owner of Elite Eye Care, New York, provides another example of AI’s potential in practice management. Her goal for this year was to increase patient volume without expanding her staff, and ChatGPT played a pivotal role in achieving this objective.
Faced with the decision between two vendors for new exam room equipment, she used AI to analyze each vendor’s pricing and financing options, weigh the pros and cons of the equipment in relation to her goals, and forecast the return on investment (ROI) for each option. This analysis enabled her to select the most suitable vendor and estimate the timeframe for recouping her investment.
Dr. Perry also leverages AI to analyze patient feedback, demographic data, and treatment outcome statistics to ensure equipment investments align with patient needs. For instance, if data reveals a high prevalence of conditions like glaucoma, AI can help justify investing in advanced glaucoma screening tools.
Summing up
The optometry landscape is evolving, driven by raised patient expectations for convenience and efficiency. Practices adapt to these changes by embracing emerging optometry trends to achieve more precise diagnostics, streamline patient journeys, enhance the exam room experience, and build trust and connection. Much of this technology is AI-based, with even more advancements on the horizon. So, optometrists implementing these solutions today are poised to secure a significant competitive advantage.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

Optometry Technology: What to Expect?
 Maria Znamenska
7 min.7 min.
Maria Znamenska
7 min.7 min.Optometry Technology: What to Expect?
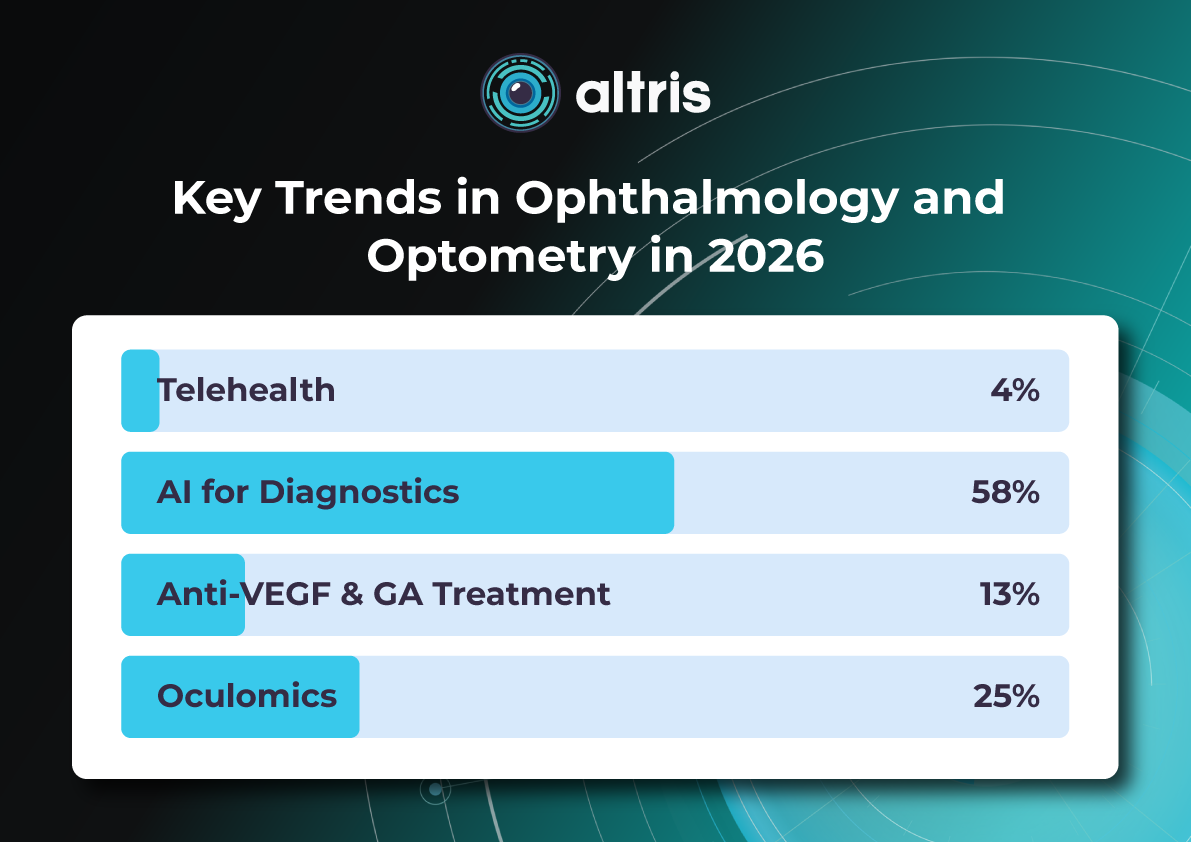
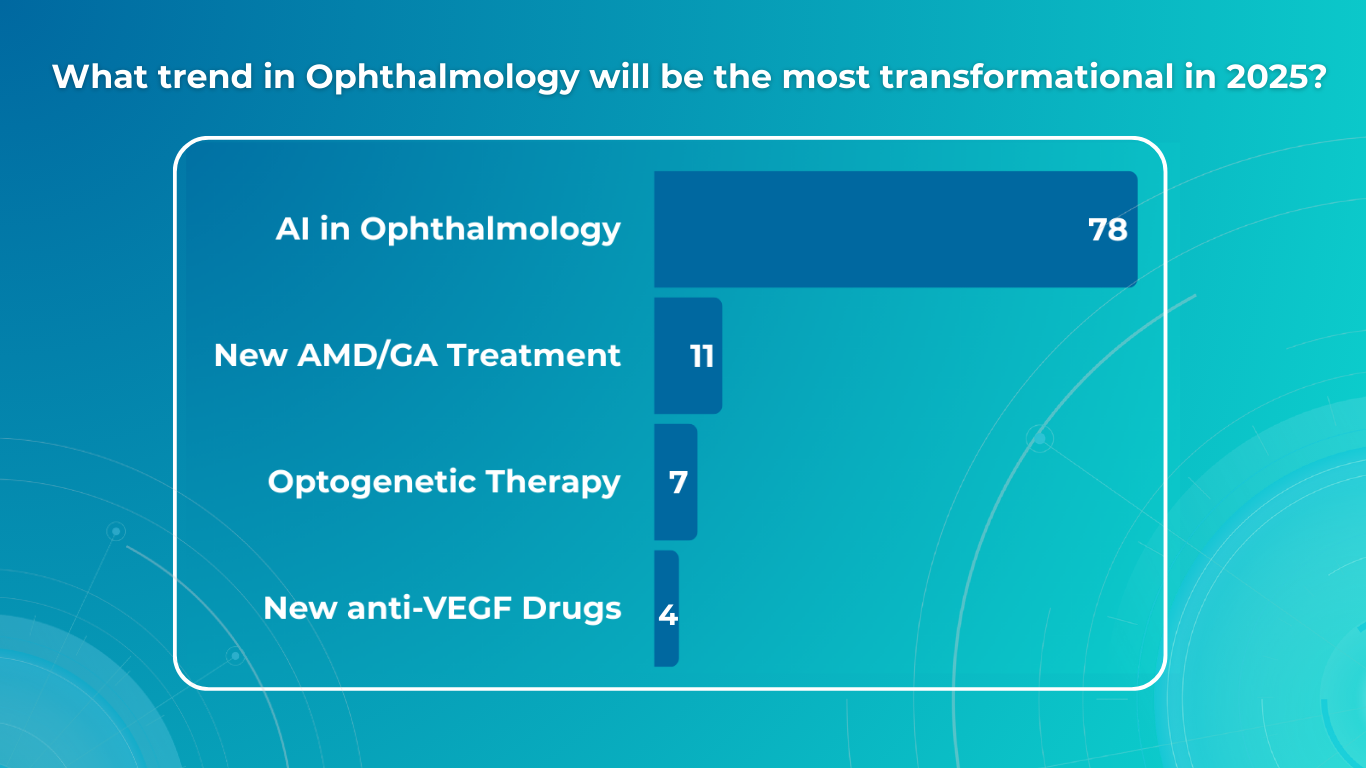
For this article, we surveyed eye care professionals on which optometry technology appears most promising to them. The answers were divided among AI for more precise diagnostics, advanced contact lenses, and new iterations of OCTs.
Of course, this is not the whole list of possible new tech in optometry, but these are the topics that draw the most attention today.
The article delves deeper into each of these technologies, as well as explores oculomics, the new way of understanding the correlation between eye pathology and overall human health.
New tech in optometry: AI for Medical Image Analysis
AI has blossomed in recent years, transforming not only how we work and relax but also how we manage our health. It’s no surprise that our survey of professionals revealed AI as the most promising technology in optometry.
The most immediate and practical AI implementation in optometry is the analysis of medical images, such as fundus photos and OCT scans.
They require no additional equipment beyond the OCT and fundus cameras many practitioners already own, are cost-effective, and add huge value to a practice.

There are many companies that detect a number of biomarkers and help with diagnostic decision-making already, and their number will only increase from year to year for several reasons:
- AI systems for medical image analysis speed up patient triage
- AI systems help to detect early, minor, and rare pathologies which sometimes can be missed
- AI systems help with complex cases when a second opinion is needed
- Quantitative analysis of biomarkers improves treatment results monitoring making it more efficient
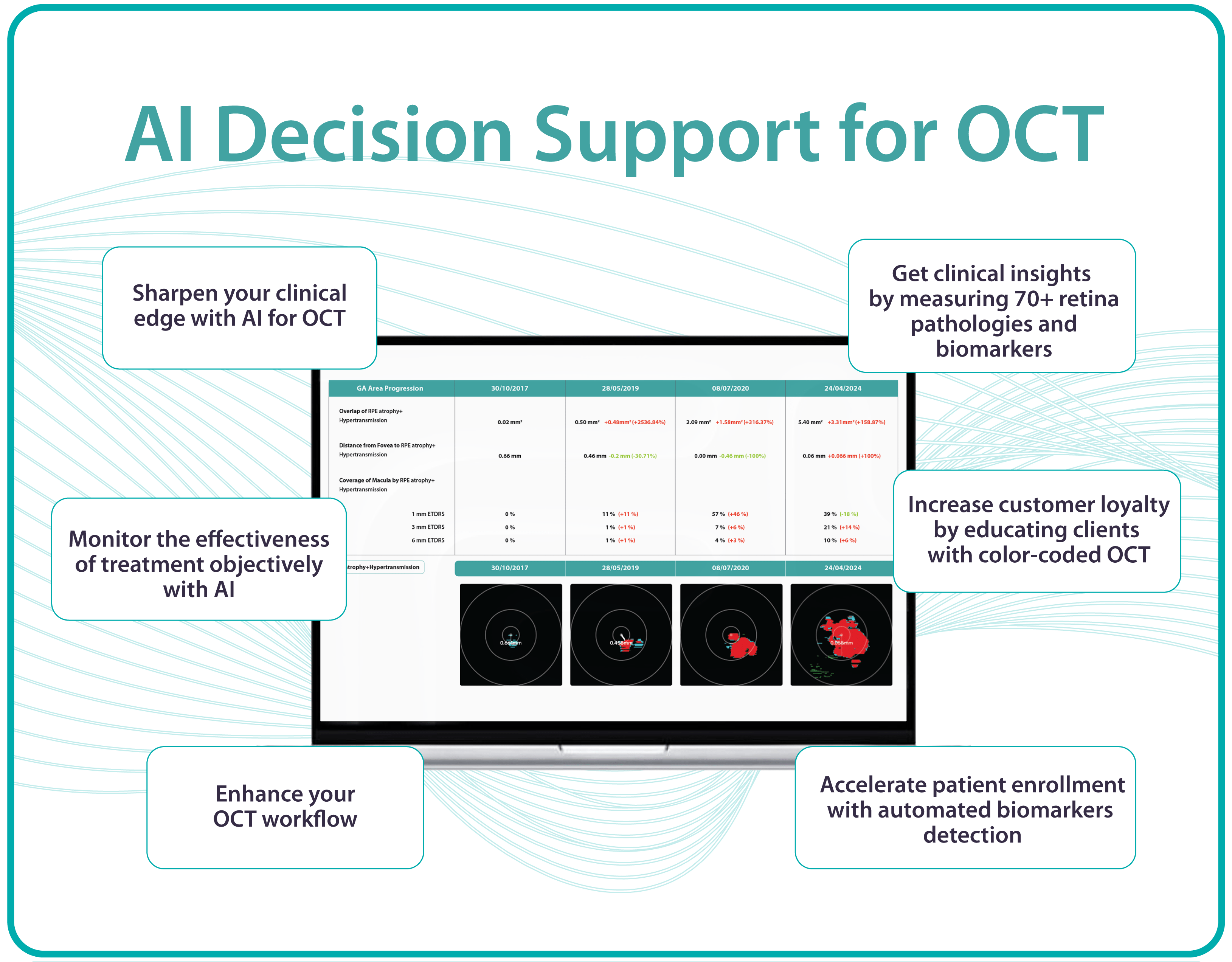
For instance, AI today can assess the early risk of glaucoma based on the GCC asymmetry measurements. Here is how AI-powered OCT workflow would look.
AI-assisted readings of OCT scans are already helping not only with pathology detection but also with the analysis of its progression or response to treatment. This represents a new approach to monitoring, where practitioners no longer need to sift through various patient notes but can directly compare reports from previous examinations and observe how, for instance, shadowing has changed in micrometers.

AI programs are becoming even more invaluable with an aging population, as diseases prevalent in older individuals become increasingly common while ophthalmology and optometry face a shortage of specialists. This situation will transform the optometrist’s role, with AI empowering practitioners with the diagnostic capabilities to manage many conditions without referral. This will benefit patients, enabling timely routine screenings and diagnoses and preventing months-long waits that can sometimes lead to irreversible blindness.
AI systems are also being implemented in ophthalmic trials for biomarker detection, exploring the relationship between imaging biomarkers and underlying disease pathways. For instance, a recent study linked levels of various cytokines, including VEGF, MCP-1, and IL-6, with specific OCT-derived biomarkers like fluid parameters and outer retinal integrity.

This significantly accelerates the research process, assisting in identifying the right target audience based on OCT scans, eliminating manual data annotation, and revealing the subtlest changes, progression or regression, and patient responses during trials.
While material advancements allow us to build more precise machines, the new tech in optometry likely won’t involve some unheard-of device. Instead, AI software will enable us to extract the maximum potential from the technologies we already use.
New Tech in Optometry: New Iterations of OCT
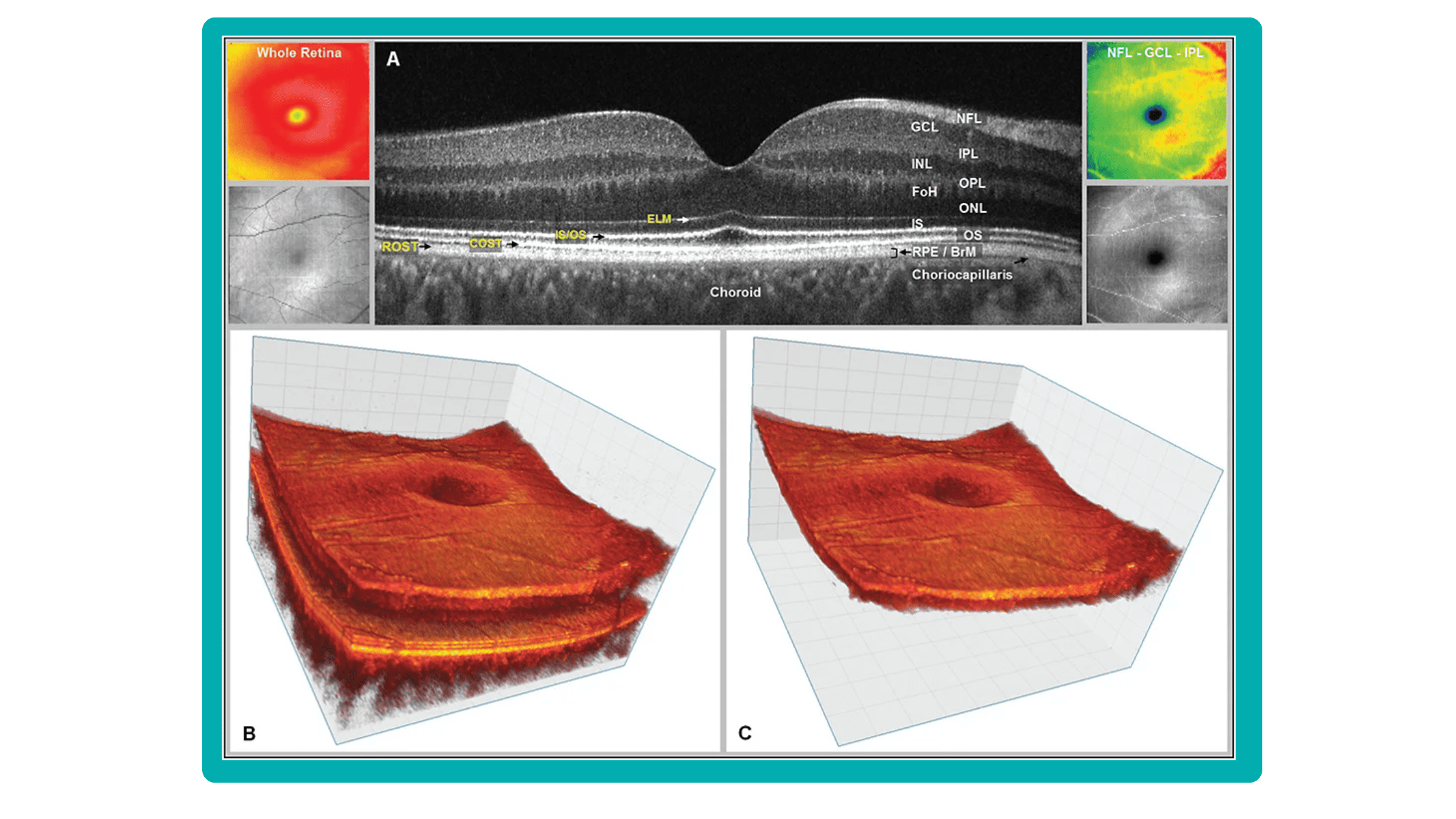
Even though OCTs entered the market relatively recently, they swiftly became indispensable ancillary tests in ophthalmic practice for many professionals. The primary reason is their high-quality imaging of the retina, nerve fiber layer, and optic nerve, offering a near in-vivo “optical biopsy” of the retina.
However, the technology continues to evolve – partly due to technological advancements and partly due to the ability to extract even more data from OCT machines through sophisticated software.
SD-OCT is undergoing continuous development, expanding its range of applications. Multimodal imaging, which combines SD-OCT with other imaging techniques like autofluorescence and angiography, now allows for improved diagnosis and management of a wider array of diseases.
Several prominent OCT evolutions combine technological advancements and promise widespread adoption. They are:
New Tech in Optometry: En-face OCT
En-face OCT in current systems is based on software reconstruction of OCT images. Image slices are selected retrospectively from full recorded volumes or calculated by depth projection along specific depth ranges, enabling three-dimensional data visualization in a fundus projection. This technique allows the projection of specific retinal and/or choroidal layers at a given depth onto an en-face view.

While we are more accustomed to working with cross-sectional images (B-scans), microstructural changes and the retinal and choroidal vasculature morphology are challenging to evaluate using B-scans alone. En-face OCT offers numerous advantages, including the ability to precisely localize lesions within specific subretinal layers using their axial location on OCT cross-sections and to register projected OCT images to other fundus imaging modalities using retinal vessels as landmarks.
Currently, en-face OCT is being applied to various specialized areas within the eye, encompassing the anterior segment, glaucoma, infectious diseases, and the retina.
Optometry Technology: SS-OCT
Like SD-OCT, swept-source OCT (SS-OCT) utilizes Fourier domain technology to optimize higher-quality wavelength transduction within the frequency domain. This enables rapid sweeping scan patterns across a broad bandwidth.
However, instead of a broad-bandwidth light source projected all at once, as in SD-OCT, SS-OCT employs a single tunable laser that sweeps through different frequencies to cover the entire spectrum swiftly. The light reflected from the eye is captured by a photodetector significantly faster than the charge-coupled device (CCD) camera used in SD-OCTs. This difference translates to a faster scanning speed of up to 400,000 axial scans per second, eliminating the typical depth-dependent signal drop-off associated with SD-OCT. Additionally, the faster scanning speed reduces image distortions caused by eye movements and allows for wider B-scans, facilitating widefield imaging.
Furthermore, many SS-OCT systems utilize a light source centered at an approximately 1050 nm wavelength, providing better tissue penetration than SD-OCT. This allows for visualization of structures like the choroid, lamina cribrosa, and structures at the anterior chamber angle. This enhanced penetration is crucial in diseases like Central Serous Chorioretinopathy, where evaluating the entire thickness of the choroid can be challenging.

Moreover, volumetric analysis of the choroid and various pathological features can aid in monitoring the progression of Wet AMD, CSCR, and Diabetic Retinopathy, as well as assessing the response to treatments such as anti-VEGF agents, laser photocoagulation, and photodynamic therapy (PDT).
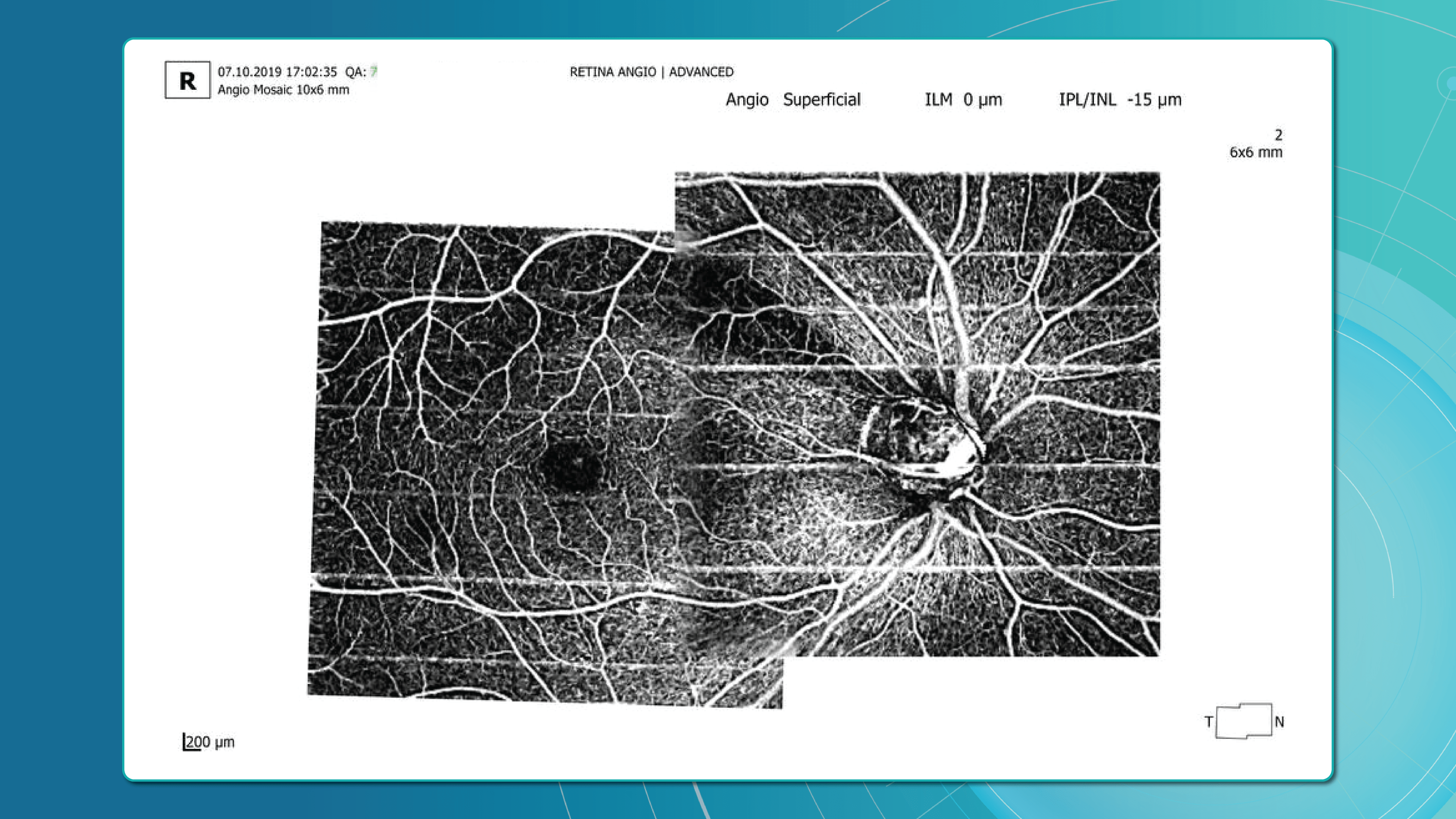
Optometry Trends: OCT Angiography
Given that many ocular diseases are associated with vascular abnormalities, the ability to visualize and quantify blood flow in the eye is crucial. Traditionally, fluorescein angiography (FA) and indocyanine green angiography (ICGA) have been used for this purpose, but these procedures require intravenous injection of contrast agents, which is not only time-consuming but may lead to allergic reactions or potentially serious side effects.
OCTA, on the other hand, produces high-resolution, 3D angiograms of the retinal and choroidal vascular networks, taking advantage of the eye’s unique characteristic as the only organ allowing noninvasive, direct observation of its blood vessels’ structure and function. OCTA detects blood flow using intrinsic signals to capture the location of blood vessels. While it has limitations such as insensitivity to leakage and a relatively small field of view, the development of OCTA has the potential to significantly enhance our understanding of the eye’s physiology and pathophysiology, providing depth-resolved angiographic maps of the tissue’s vascular structure down to the capillary level.
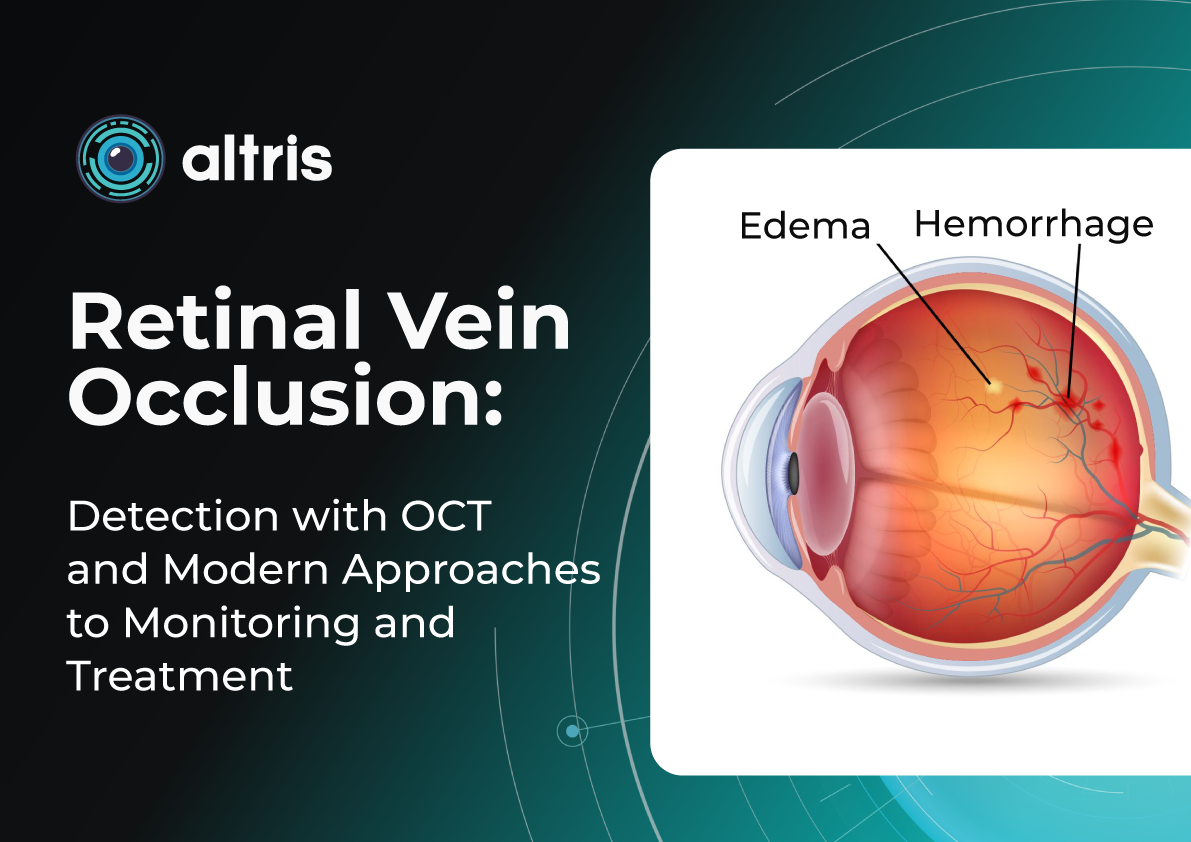
OCTA is particularly valuable in clinical settings where pathologies like diabetic retinopathy, age-related macular degeneration, retinal vein occlusions, and macular telangiectasia are frequently encountered. These conditions often alter blood flow or the blood vessels themselves in the retina, making imaging these vessels essential for diagnosis and management.

Wide-Field and Ultrawide-Field OCT (WF-OCT and UWF-OCT)
While OCT is a powerful ocular imaging tool, it has traditionally been limited by a relatively narrow field of view (FOV) – typically around 20 degrees × 20 degrees. To address this limitation, two advancements have emerged:
- Wide-field OCT (WF-OCT) with an FOV of approximately 60-100 degrees captures the retina’s mid-periphery up to the posterior edge of the vortex vein ampulla.
- Ultrawide-field OCT (UWF-OCT) with an FOV of up to 200 degrees, mapping the far periphery of the retina, including the anterior edge of the vortex vein ampulla and beyond.
WF-OCT provides additional information compared to routine 6-9 mm scans in conditions such as diabetic retinopathy (DR), central serous chorioretinopathy (CSCR), polypoidal choroidal vasculopathy (PCV), peripapillary choroidal neovascular membrane (CNVM), or uveitic entities. It facilitates easier visualization of anatomical details of peripheral retinal changes like ischemic areas in DR, retinal vein occlusions, or sites of retinal breaks, peripheral retinal detachment, retinoschisis, and choroidal lesions (melanoma, nevus, hemangioma, choroidal metastasis).
As with other OCT iterations, WF and UWF OCT will likely provide the most significant insights when routinely combined with other modalities, such as OCT angiography.

New Tech in Optometry: Advanced contact lenses
In our lifetime, contact lenses have evolved from mere corrective devices to sophisticated optical instruments. There are several ways that contact lenses (CLs) continue to advance:
- Manufacturing optimization: Automation and robotization of the process for higher precision and a shift towards a more environmentally friendly approach.
- Design: More precise designs tailored to the wearer’s eye with the help of 3D printing.
- Material advancements: Nanotechnology/surface modifications for improved wettability, lubricity, and antimicrobial properties. Increased focus on biomimetic design.
- Technological advancements: Smart lenses with thin and ultra-thin transistors capable of reacting to or registering the wearer’s stress levels, glucose levels, etc.
Let’s take a closer look at a few examples of Smart Contact Lenses (SCLs) that combine some of the characteristics mentioned earlier.
SCLs are wearable ophthalmic devices that offer functions beyond vision correction. These devices are integrated with sensors, wireless communication components, and microprocessors to measure biological markers. They can treat ocular pathologies by delivering drugs, light, heat, and electrical stimulation, or they can aid in diagnosing. Currently, some SCLs can help manage glaucoma, cataracts, dry eye syndrome, eye infections, and inflammation. In development are lenses to treat age-related macular degeneration (AMD), diabetic retinopathy (DR), retinitis, and posterior uveitis. An artificial retina (retinal prosthesis) is in its early developmental stage, with the potential to restore vision to some degree for specific types of blindness caused by degenerative diseases.

Scientists from the School of Medical Sciences in New South Wales have implanted epithelial stem cells (ESCs) from a healthy eye into a contact lens. This innovation has shown promise in repairing vision loss caused by a damaged cornea. In another breakthrough, scientists from Oregon State University have utilized ultra-thin transistor technology to design SCLs that can monitor the wearer’s physiological state. While this futuristic contact lens is still in the prototype phase, several biotech companies have already expressed interest in its development.
Smart lenses also show great promise in drug delivery. One of the main challenges with eye drops is their low bioavailability (less than 5%), primarily due to high tear turnover rates, blinking, nasolacrimal drainage, non-productive absorption by the conjunctiva, and the cornea’s low permeability. Therefore, improving bioavailability by increasing the drug’s residence time on the ocular surface remains a critical research focus.
Additionally, drug delivery via SCLs can offer more precise dosing. With traditional eye drops, dosage accuracy relies on the patient’s ability to tilt their head and squeeze the inverted bottle correctly, leading to inconsistent application. Consequently, compliance rates for eye drops are low. In contrast, the drug delivery process with SCLs involves lenses loaded with medication for a day or several days, potentially enhancing compliance, especially for individuals accustomed to wearing contact lenses as part of their routine.

Just as artificial intelligence is merging with ophthalmic devices for detection and analysis, opening new possibilities, optometry trends are also venturing contact lenses into the multidisciplinary field of theranostics, which combines therapeutics and diagnostics. This field is uncovering new avenues of research, shedding light on disease mechanisms, and driving drug and medical device development. Theranostics leverages knowledge and techniques from nanotechnology, molecular and nuclear medicine, and pharmacogenetics to achieve goals such as in vitro diagnostics and prognostics, in vivo molecular imaging and therapy, and targeted drug delivery. This approach is shifting patient care towards proactive strategies and predictive treatments.
Optometry Technology: Oculomics
For decades, researchers have sought to measure retinal changes to identify ocular biomarkers for systemic diseases, a field now known as oculomics.
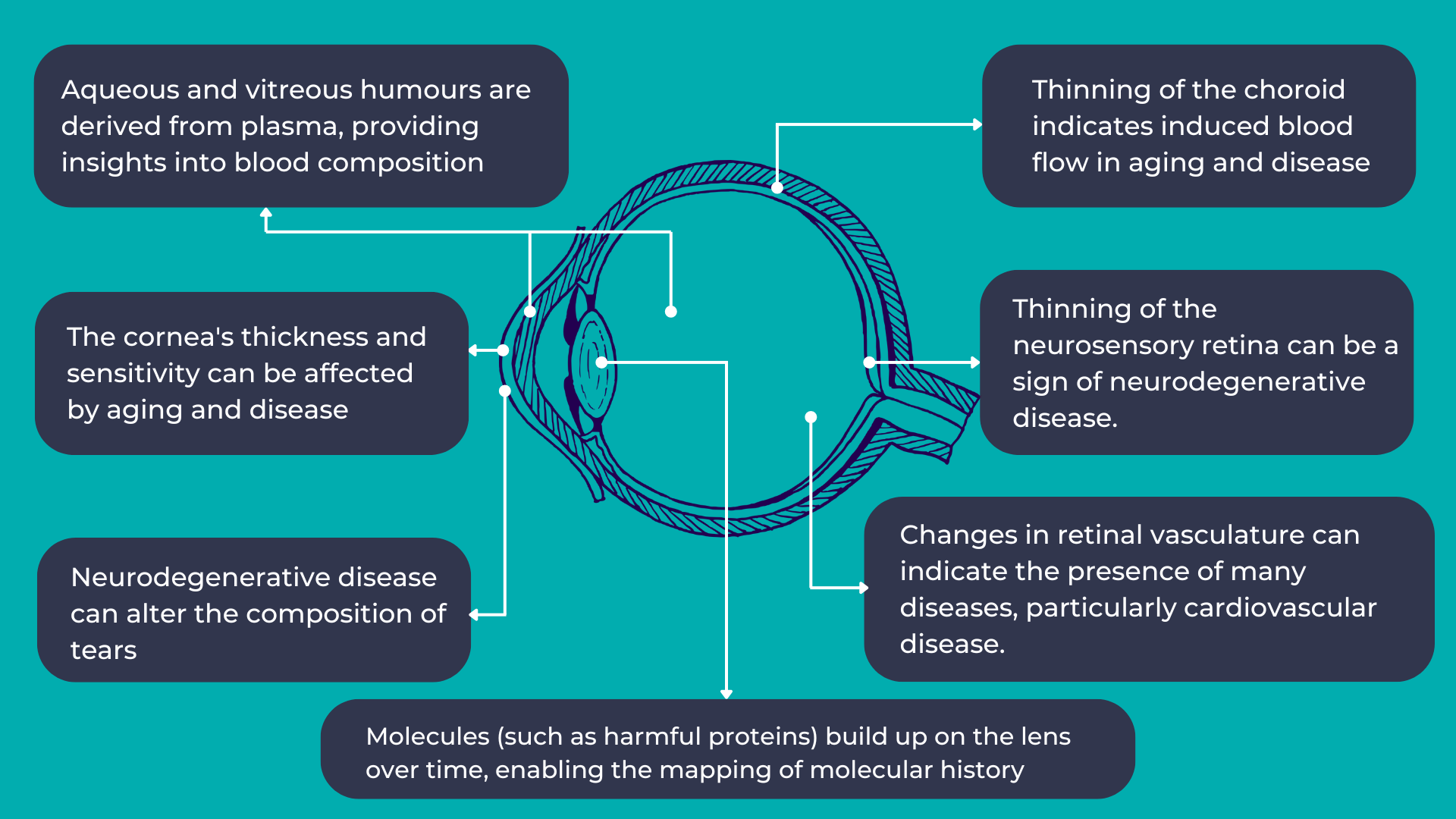
As mentioned earlier, the eye provides a unique opportunity for direct, in vivo, and often non-invasive visualization of the neurosensory and microvascular systems:
- The eye shares a common embryological origin with the brain, and the neurosensory retina and optic nerve are considered extensions of the brain, allowing direct observation of the nervous system.
- Due to the length and continuity of the visual pathway, along with trans-synaptic degeneration mechanisms, damage to the central nervous system often manifests as changes in the inner retina.
- The blood-retina barrier, similar to the blood-brain barrier, selectively allows the transport of essential substances to these metabolically active structures.
- The aqueous and vitreous humors are plasma-derived and transport lipid-soluble substances through diffusion and water-soluble substances through ultrafiltration.
- The lens, which grows continuously throughout life, accumulates molecules over time, providing a potential map of an individual’s molecular history.

The link between the eye and overall human health is not new. However, with the increasing availability and complexity of large, multimodal ocular image datasets, artificial intelligence-based ocular image analysis shows great promise as a noninvasive tool for predicting various systemic diseases. This is achieved by evaluating risk factors, retinal features, and biomarkers. Thanks to the massive datasets generated through recent ophthalmic imaging, which are now being used for deep learning and AI training, oculomics is starting to yield more precise answers. For example, the NHS alone has been conducting eye tests for over 60 years, resulting in databases containing millions of images, complete with patient records and long-term health outcomes. These datasets have been fed into AI algorithms, leading to models that can already predict cardiovascular risk factors with accuracy comparable to the current state-of-the-art methods.
It’s a significant opportunity because, with the aging population, a primary healthcare focus will be not only extending lifespan longevity but also maintaining crucial healthspan functions. The primary obstacles to both longevity and healthspan are chronic diseases, referred to as the “Four Horsemen of Chronic Disease” (Cardiovascular disease, Cancer, Neurodegenerative disease, and Metabolic disease). Many of these can be, if not entirely prevented, at least minimized in terms of progression through timely detection and intervention.
One major advantage of discovering biomarkers that can predict diseases is that eye screenings are generally less intimidating than other procedures. For example, a person might regularly visit an optometrist for prescription glasses but avoid routine cervical screenings. A less anxiety-provoking and familiar procedure could significantly impact healthcare engagement. Such screenings could also make a substantial difference for chronic conditions like dementia, diabetes, and cardiovascular disease, which constitute a significant portion of the “burden of disease.”
Summing up
Artificial intelligence has already significantly impacted our lives. It holds immense promise in optometry technology, as its primary capability—analyzing massive datasets—aligns perfectly with eye care, where thousands of images are generated daily. Training on such vast amounts of data will lead to breakthroughs in pathology and biomarker detection and their correlation with overall human health. It will enable us to take a giant leap towards proactive and predictive medicine, helping our patients live longer, healthier lives.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

Altris Announces Appointment of Grant Schmid as a VP of Business Development
 Altris Inc.
26.08.20241 min.
Altris Inc.
26.08.20241 min.Altris AI Announces the Appointment of Grant Schmid as the VP Business Development
Altris AI, a leading AI software provider for OCT scan analysis, announces the appointment of Grant Schmid as the Vice President Business Development. Mr. Schmid is a proven leader in the eye care industry and has solid experience that will help him establish new partnerships for the company and lead corporate sales.
The recent surge in AI (artificial intelligence) applications across industries has transformed the technology landscape, especially in healthcare. While AI companies have existed for years, the explosion of tools like ChatGPT has popularized the integration of AI in everyday processes.
Grant was drawn to Altris AI for its focus on harnessing AI capabilities to assist doctors in making faster and more informed decisions.
According to Mr. Schmid,
“Healthcare professionals are inundated with more data than most other professions, particularly in the eye care segment. Eye care specialists are subjected to multiple tests and instruments, generating a vast amount of data that must be reviewed comprehensively. A single Optical Coherence Tomography (OCT) test can contain over five hundred thousand data points. This necessitates that doctors carefully analyze results from various tests, often overlapping with different devices, which can be time-consuming and detract from the time they have with their patients.”
At Altris AI, the mission is not to replace the vital human connection in medicine but to enhance it.

Grant also remarked that,
“Some AI companies are positioning their products as replacements for human doctors, which undermines the essential aspects of patient care. Patients need to feel heard, and doctors choose this profession to help individuals. Altris AI enables doctors to spend more time with their patients, allowing them to focus on the human aspects of care rather than getting lost in data analysis.”
About Altris AI.
Altris AI is a part of the Altris Inc. ecosystem that includes Altris AI( a standalone AI platform for OCT scan analysis that improves diagnostic decision-making for eye care specialists) and Altris Education OCT (a free mobile app for OCT education interpretation). The mission of the company is to set higher diagnostic standards in the eye care industry and improve patient outcomes as a result. To achieve this mission the company created an AI-powered platform for OCT scan analysis that detects the biggest number of biomarkers and retina pathologies on the market today: 70 + including early glaucoma. More than that, the company offers an automated quantitative analysis of biomarkers and a progression analysis module for monitoring treatment results more efficiently.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes only, not for clinical diagnosis purposes.
-

Increasing Referral Efficiency in Eye Care: Addressing Data Gaps, Wait Times, and more
 Maria Martynova
04.07 20237 min read
Maria Martynova
04.07 20237 min readOphthalmology has the highest average number of patients waiting, but up to 75% of patients make preventable trips to eye hospitals and general practitioners. Some of these patients are referred by optometrists who, more often than not, receive no feedback on the quality of their referrals, perpetuating this cycle. Optometry referral is puzzling for both primary and secondary education. This article examines the referral procedure and potential solutions for increasing referral efficiency in eye care that practitioners can implement.
More than 25% of U.S. counties lack a single practicing eye care provider, and the situation isn’t unique to the U.S. In the UK, ophthalmology has been the most overburdened healthcare sector for some time. With a globally aging population and an increasing prevalence of age-related diseases, ensuring accessible eye care is crucial. Unfortunately, the reality is quite the opposite. One contributing factor is the high number of failures in the referral process.
How did we arrive at this point, and what can be done to improve it?
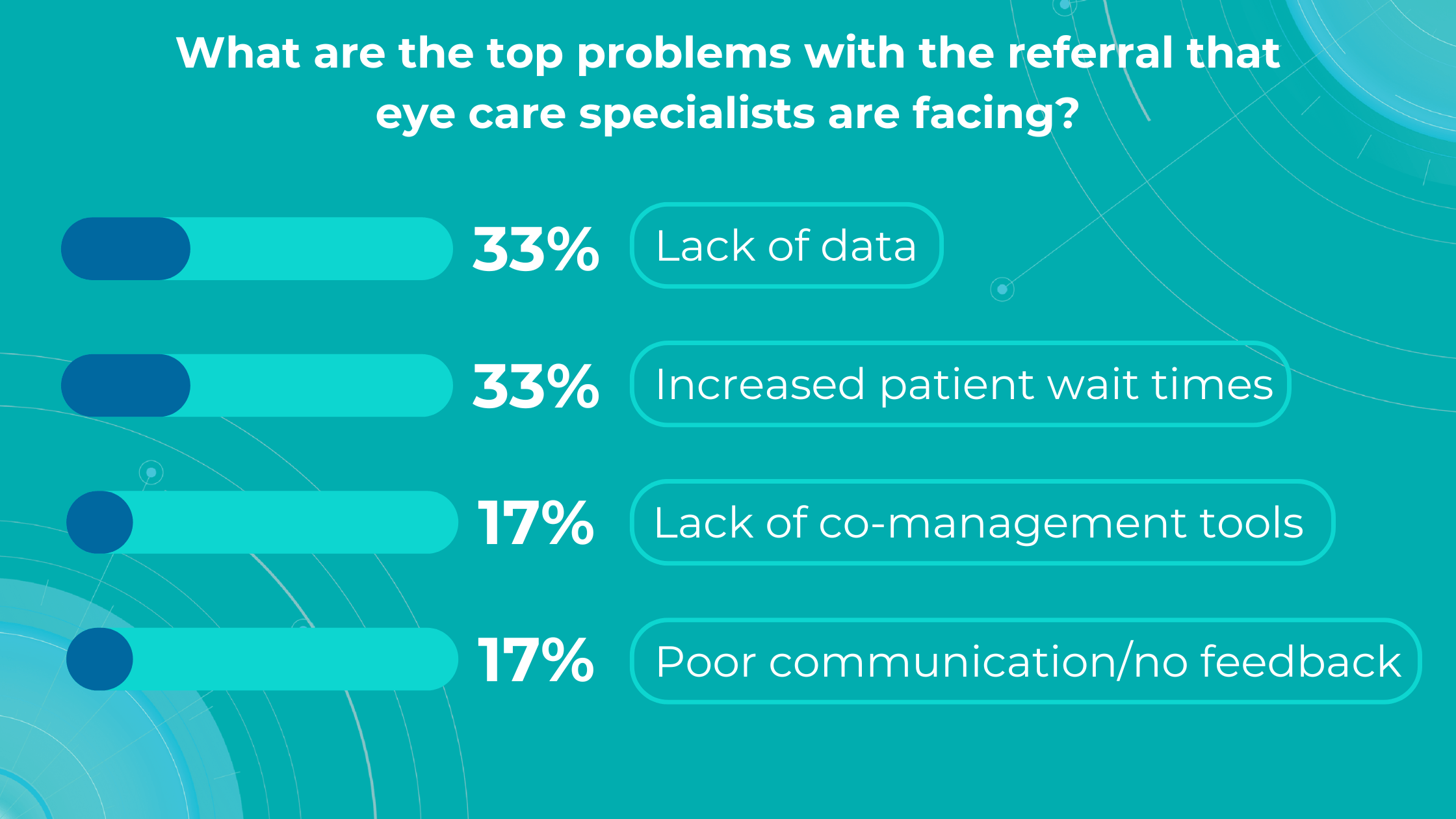
Altris AI’s survey identified a lack of data and increased patient wait times as the top problems with referrals for practitioners, while lack of co-management tools and poor communication/feedback ranked lower.

Let’s dive into more details:
Optometry referral: top problems
-
Lack of diagnostic data
The ultimate goal of optometry referral is to ensure patients receive appropriate treatment for their specific pathology or confirmation of its absence. The receiving specialist’s first step is to review the referral report, making its completeness and clarity paramount. While there is a clear need for specialised assessment and treatment, almost 80% of those attending eye casualty do not require urgent ophthalmic attention following triage, and up to 60% of patients are seen and discharged on their first visit.
In eye care, both text information and accompanying images are crucial in ensuring efficient and accurate diagnoses.
However, handwritten and fragmented data continue to pose significant challenges in the patient referral process. Despite the prevalence of electronic health records (EHRs), over half of referrals are still handled through less efficient channels like fax, paper, or verbal communication. This can lead to fragmented or doubled patient data, potential gaps in care, and delays in treatment.
The study on the Impact of direct electronic optometric referral with ocular imaging to a hospital eye service showed that, given some limitations, electronic optometric referral with images to a Hospital Eye Service (HES) is safe, speedy, efficient, and clinically accurate, and it avoids unnecessary HES consultations.

Direct electronic referrals with images reduced the need for hospital eye service appointments by 37% compared to traditional paper referrals. Additionally, while 63% of electronic referrals led to HES appointments, this figure was 85% for paper referrals.
While incorporating images like OCT scans can significantly enhance understanding, some subtle or early-stage pathologies might still be overlooked. This is where detailed and customized reports become invaluable.
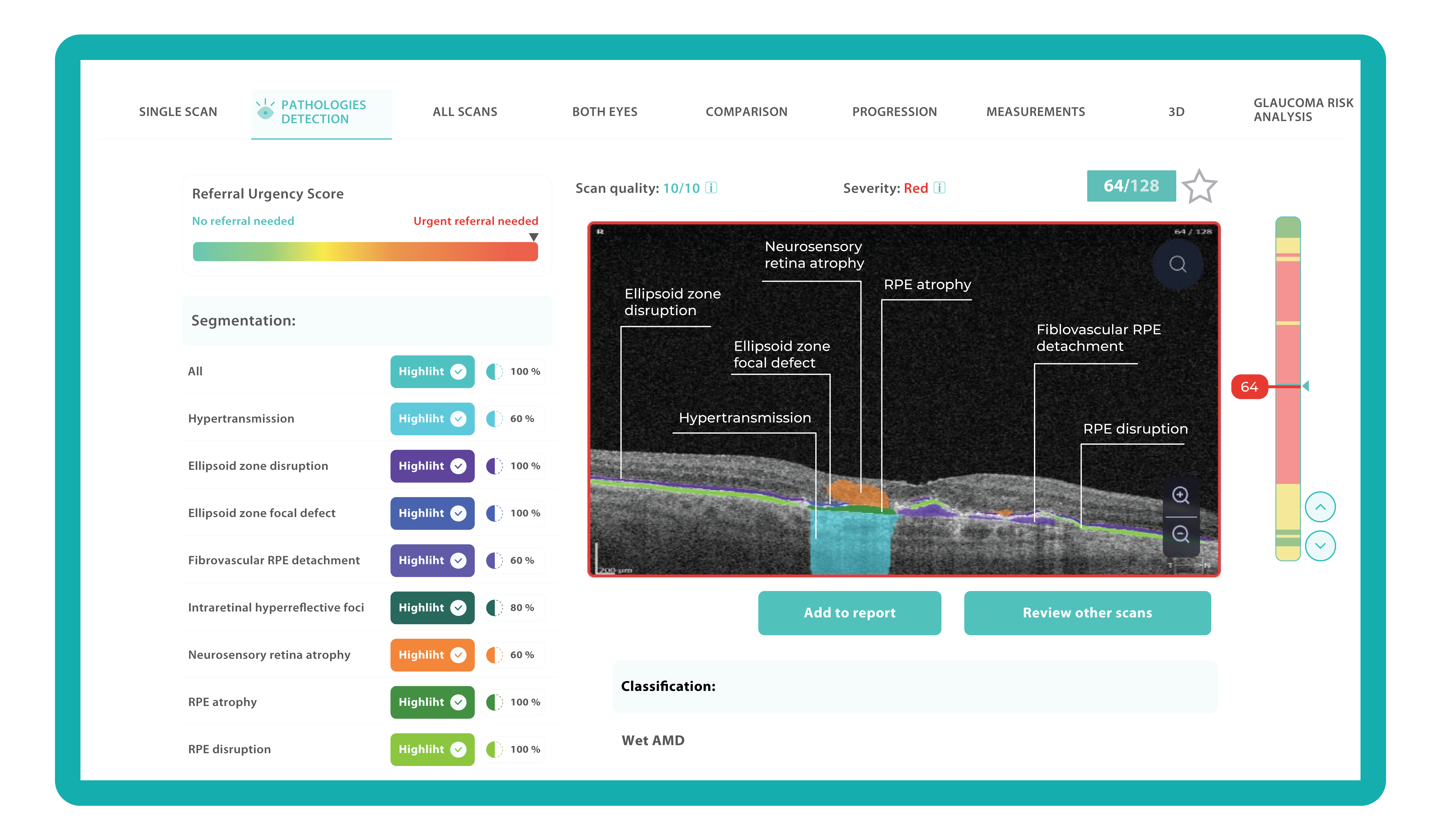
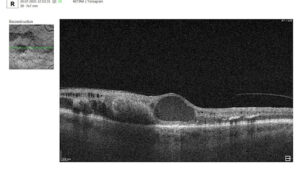
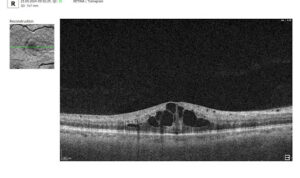
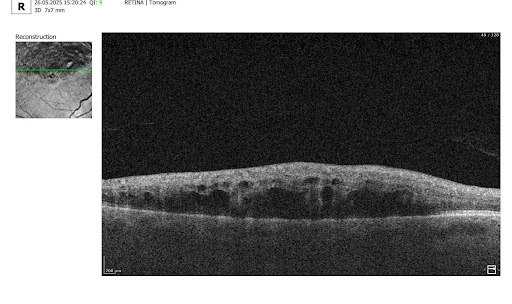
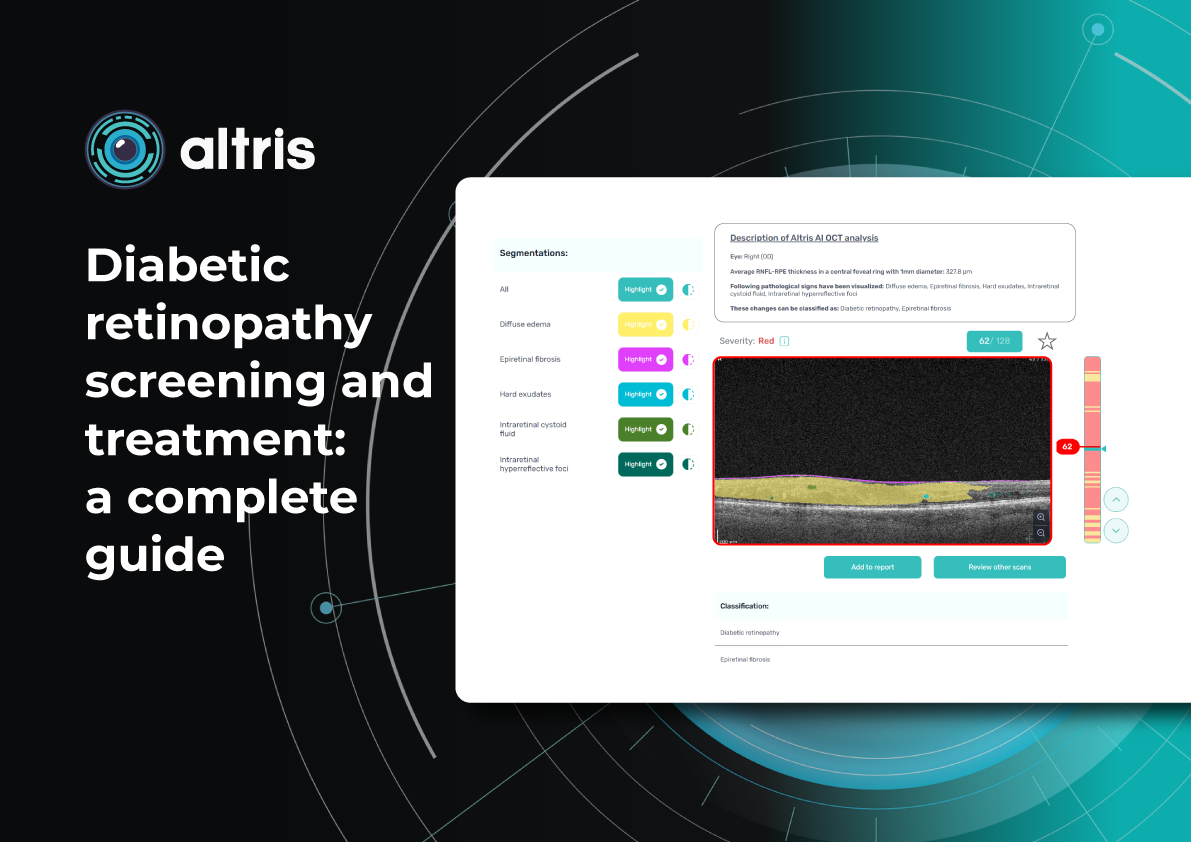
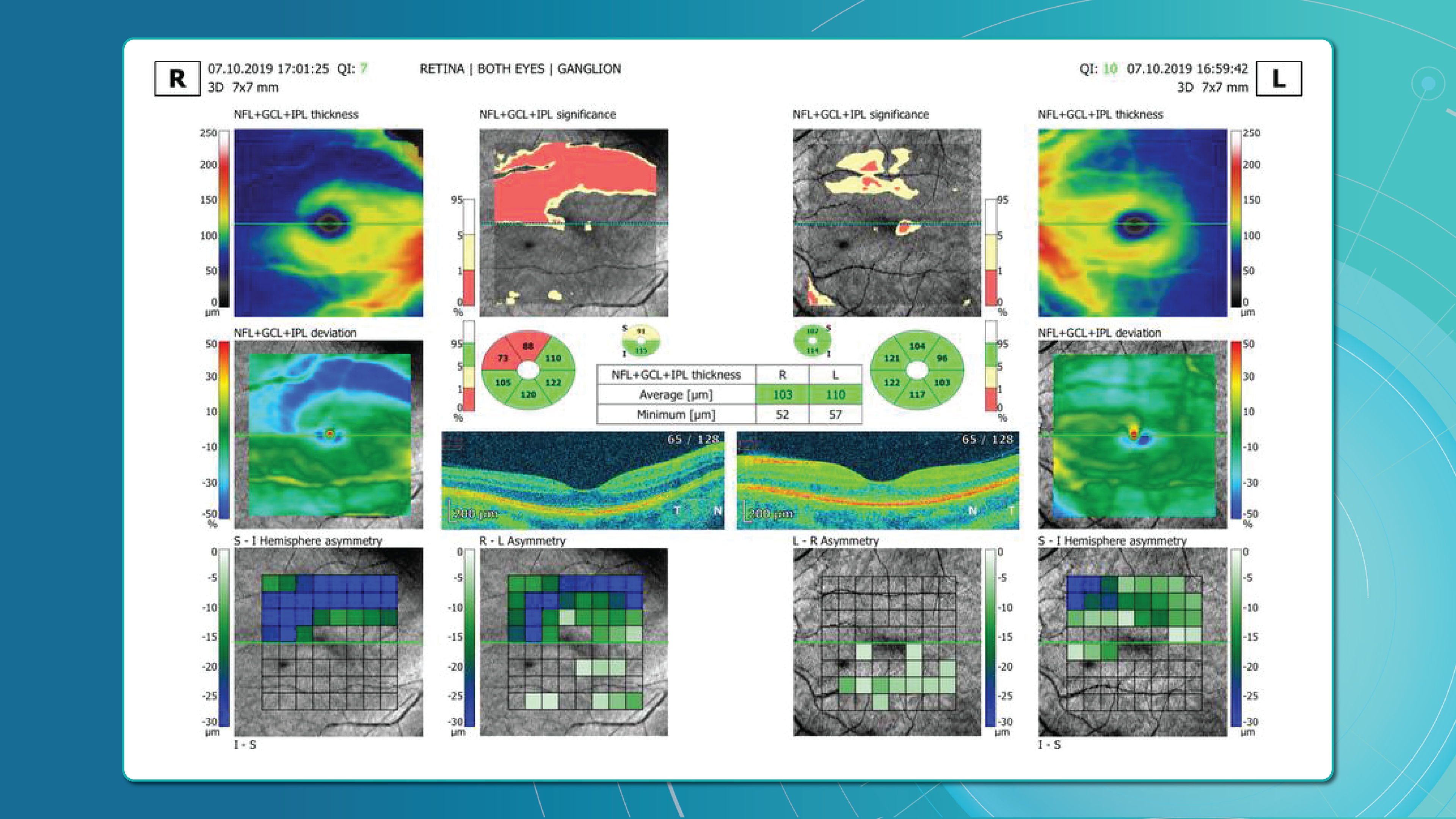
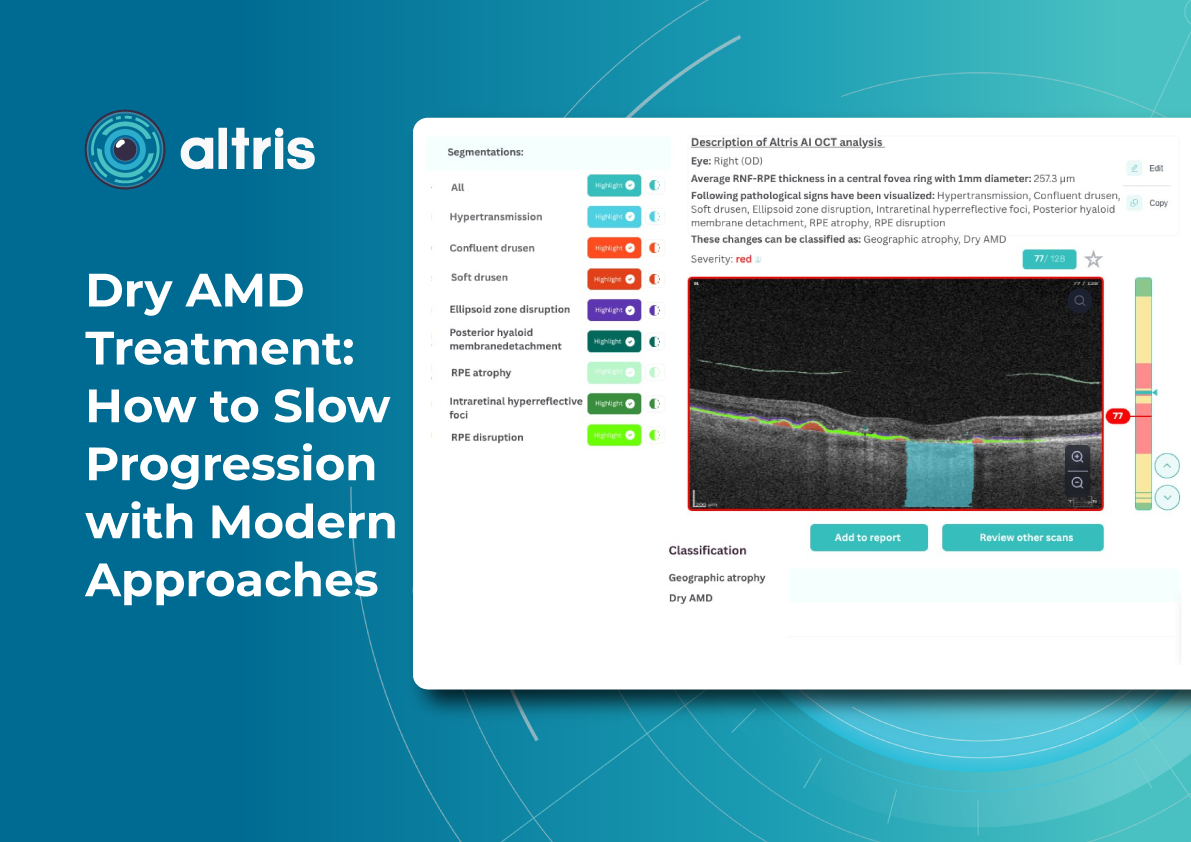
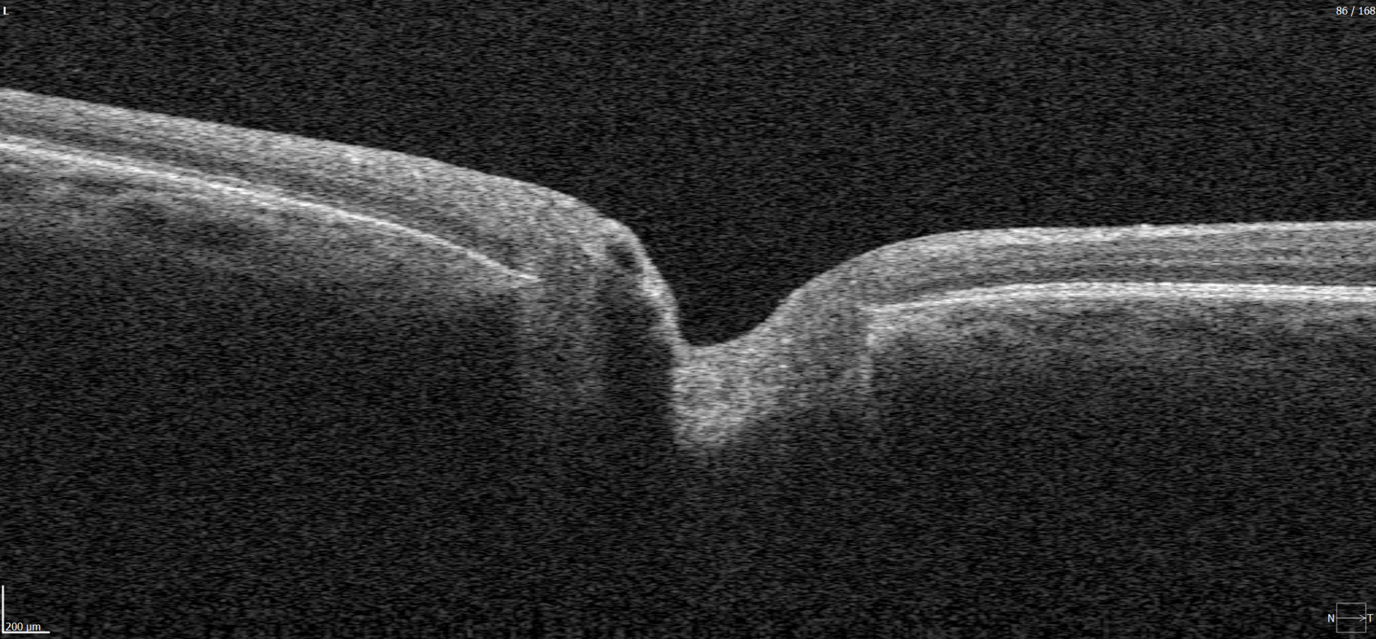
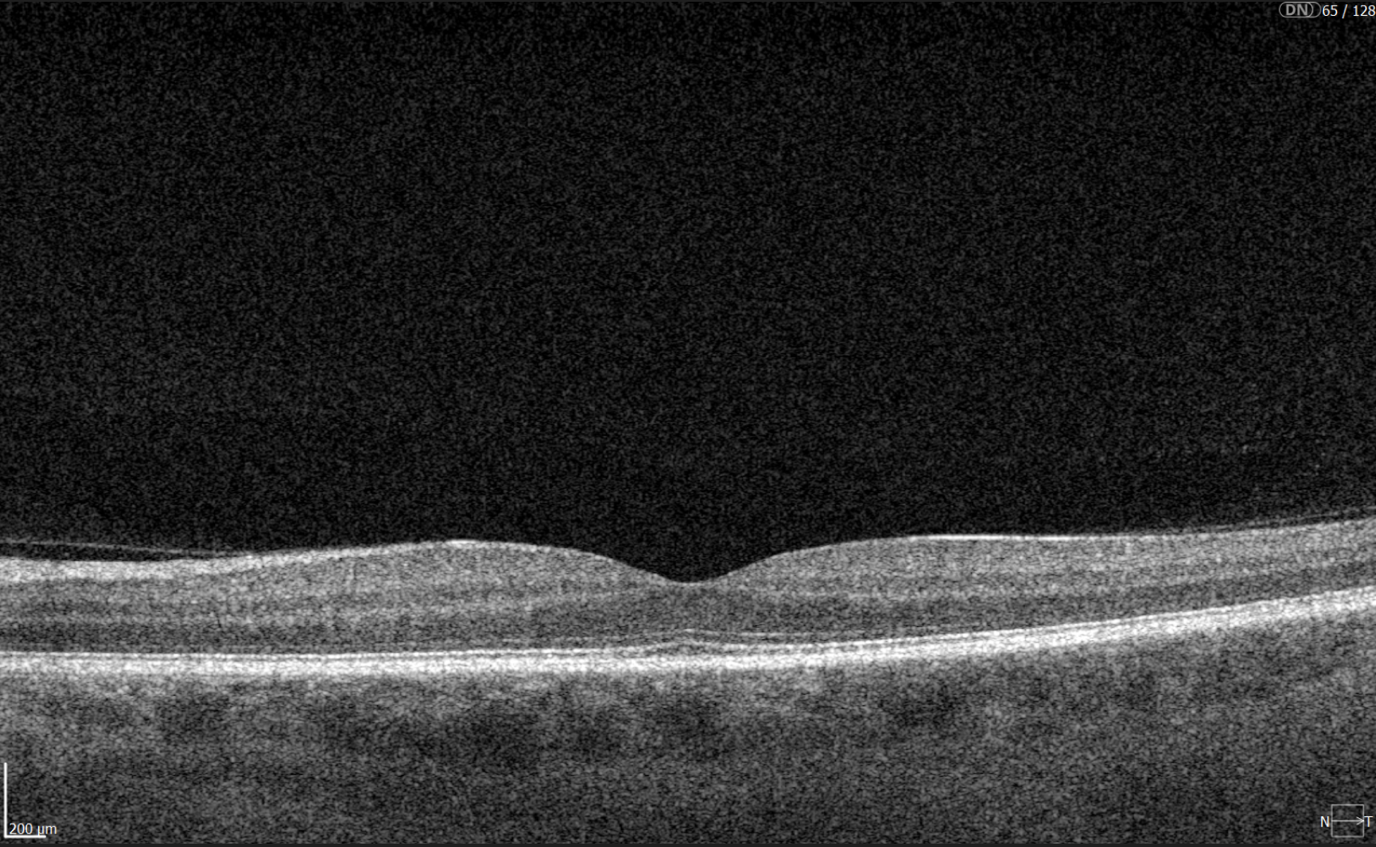
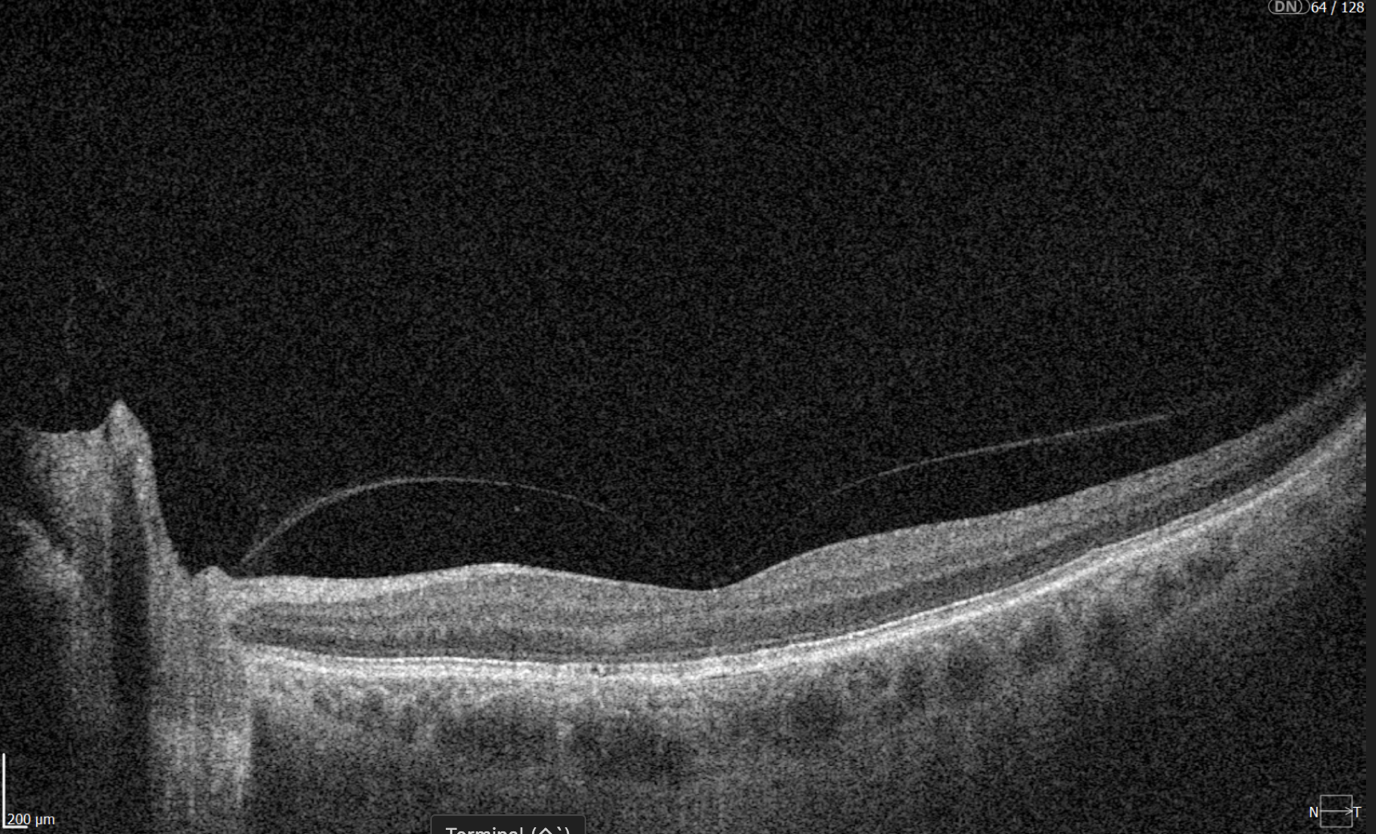
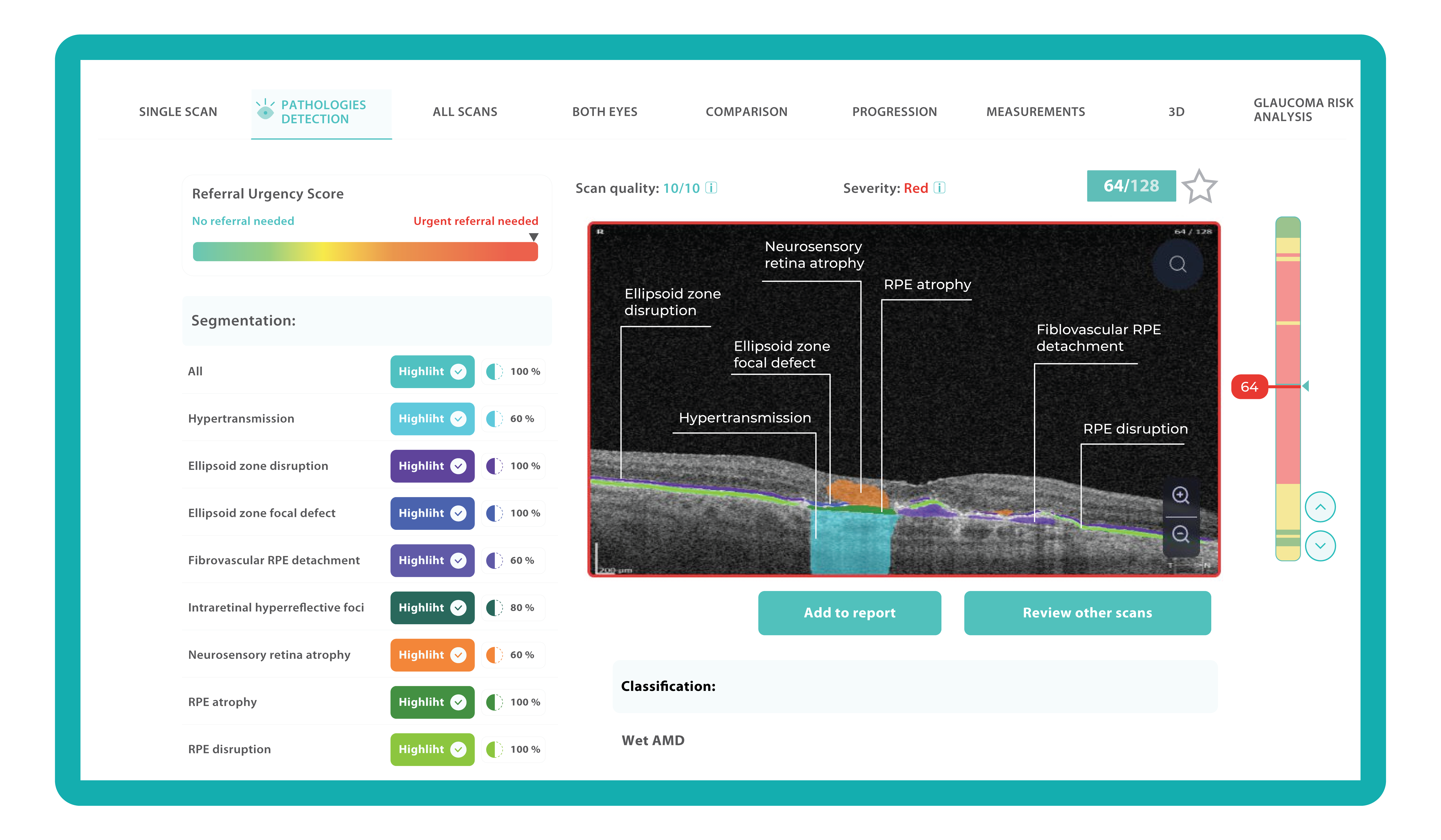
To illustrate the point, here is a handwritten referral compared to one of the types of customised OCT report from the Altris AI system, a platform that automates AI-powered OCT scan analysis for 70+ pathologies and biomarkers. This screenshot, in particular, shows segmented retina layers and highlights biomarkers of Dry AMD alongside a comparison of the patient’s macular thickness over visits.
-
Lack of experience and access to a second opinion
Research reveals a notable inverse relationship between clinician experience and the frequency of false-positive referrals in optometry, echoing findings in other medical fields where diagnostic proficiency typically improves with experience. This highlights the importance of recognizing the learning curve inherent in optometric practice and supporting less experienced practitioners.
The challenge is amplified by the fact that optometrists often practice in isolation, lacking the immediate professional support network available to their hospital-based counterparts. Unlike colleagues in hospital settings who have ready access to peer consultation for other opinions or guidance, optometrists often face limited opportunities for collaborative decision-making and skill development.
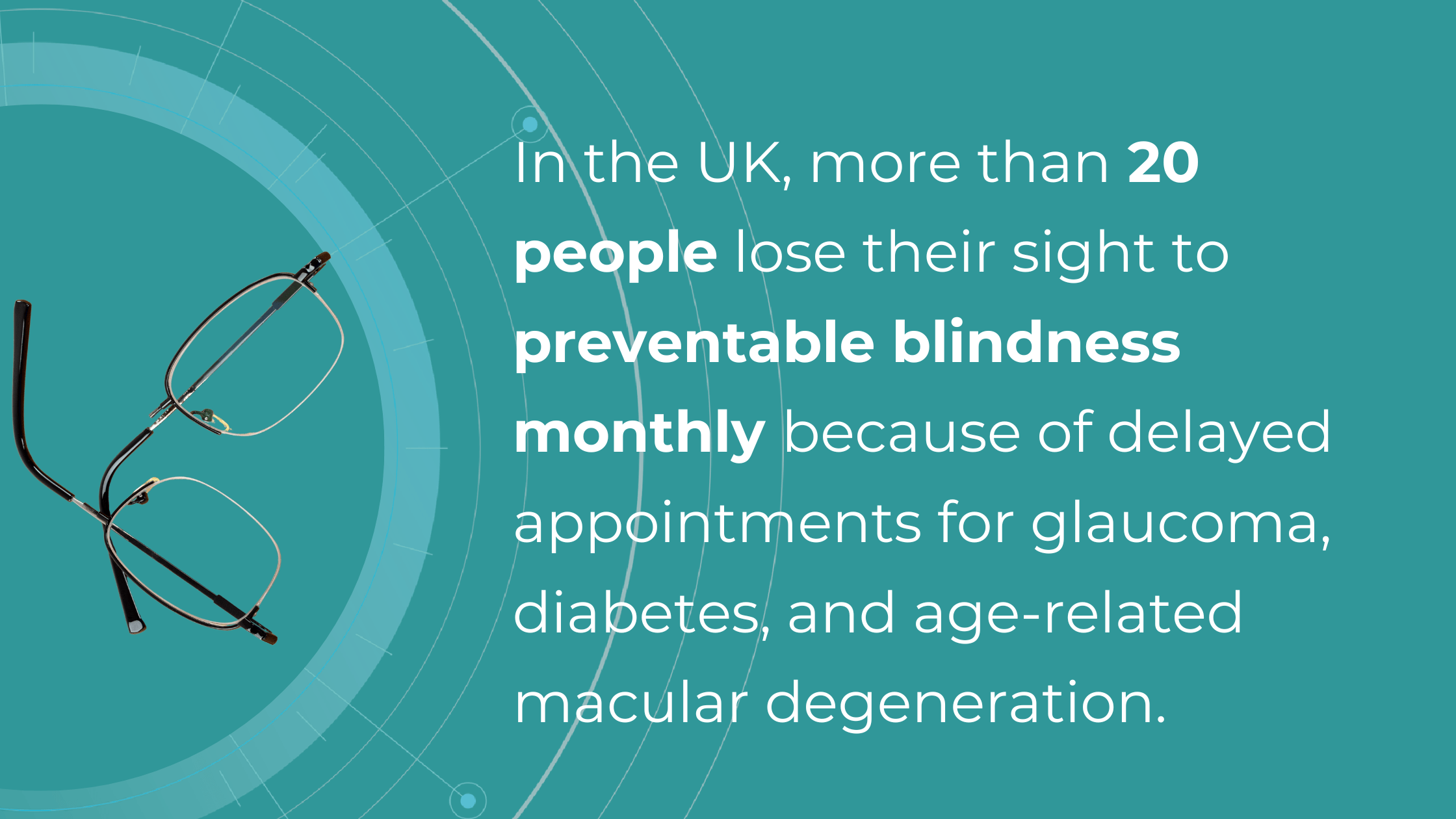
Another problem specialists often face is a lack of confidence in diagnosing, which may or may not be linked to experience. Knowing that their patients could potentially suffer irreversible vision loss from a pathology not yet detected during an exam, they often err on the side of caution and refer to a hospital. While this “better safe than sorry” approach is understandable, it places a significant burden on hospitals, extending wait times for those already at risk of blindness.
These concerns primarily revolve around glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy (DR). AI can help identify these and other eye diseases at their earliest stages during routine visits. Some retinal changes are so minute that they escape detection by the human eye, making the program’s ability to detect tiny retinal changes invaluable.
Another significant benefit of AI systems lies in their approach to OCT analysis for glaucoma. Traditional methods rely on normative databases to assess retinal normality, but these databases are often limited in size and represent a select group of individuals. This can result in missed diagnoses of early glaucoma in those who deviate from the “norm” or unnecessary referral from optometry to ophthalmology for those who don’t fit the “normal” profile but have healthy eyes. AI can overcome this limitation by providing more personalized and comprehensive analysis.
-
Increased wait times for patients with eye doctor referral

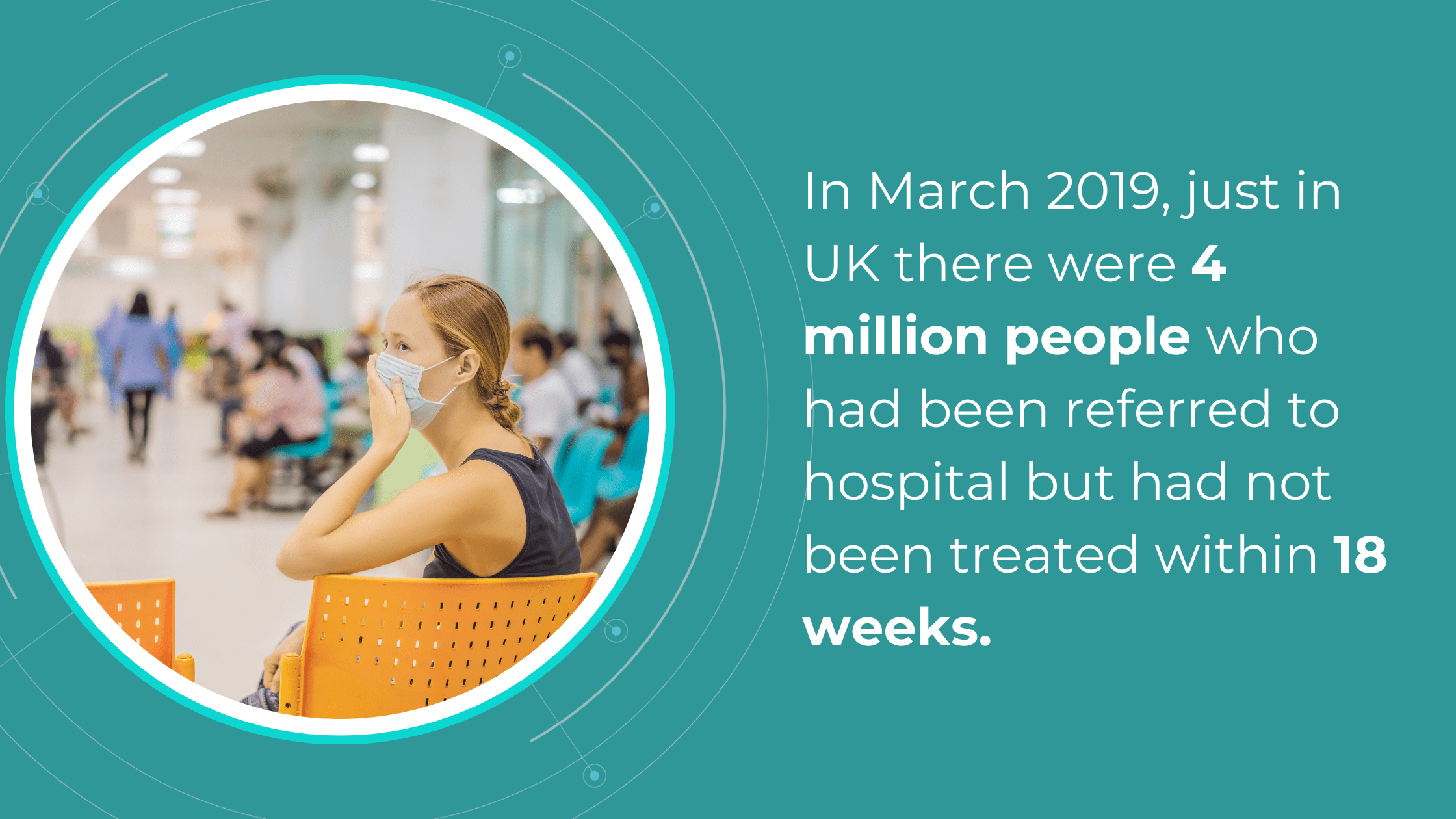
The National Health Service (NHS) is grappling with significant backlogs in ophthalmology services, which account for nearly 10% of the 7.8 million patients awaiting treatment.
The consistently high average number of patients waiting per trust in Ophthalmology, with high follow-up waitlists, delays care that poses substantial risks. The Royal College of Ophthalmologists reported that the risk of permanent visual loss is nine times higher in follow-up patients than in new patients. With 30% more patients on ophthalmology waitlists than pre-pandemic, the number of people at risk of sight loss may have increased.

Community Eyecare (CHEC), a provider of community-based ophthalmology services, received around 1000 referrals per week before the pandemic, further highlighting the strain on the system.
An analysis of electronic waitlists revealed that administrative issues, such as deceased patients or those already under care remaining on the list, artificially inflate wait times by up to 15%.
Improving administrative processes and reassessing referrals for appropriateness could help address this problem. Additionally, interim optometric examinations could revise referral information or determine the necessity of hospital visits, further reducing wait times.
Artificial intelligence can significantly speed up the screening process while reducing the controversy around diagnoses. This faster and more accurate diagnostic tool will enable more patients to be seen, allow for quicker responses to pathologies that pose a risk to eyesight, and reduce the burden on strained hospitals with needless patient referrals, as well as free up patients from unnecessary stress and wasted time.
International studies have shown that collaborative care also can increase screening and detection rates of eye disease.
-
Lack of comanagement tools for optometry referral
The increasing demand for Hospital Eye Services, projected to grow by 40% in the next two decades and currently accounting for 8% of outpatient appointments, necessitates a re-evaluation of referral pathways and comanagement strategies between optometrists and ophthalmologists.
The lack of digital connectivity between primary, community, and secondary care creates a significant barrier to effective collaboration. In many cases, optometrists cannot make direct digital referrals to Hospital Eye Service, often relying on general practitioners as intermediaries, causing delays in diagnosis and treatment.
The COVID-19 pandemic highlighted the vital role of optometrists as first-contact providers for eye health, relieving pressure on hospitals. However, better integration between primary and secondary care is essential to build upon this and create a more sustainable eye care system. The current lack of digital connectivity hinders efficient communication and impedes the timely transfer of patient records, potentially leading to unnecessary referrals and delays in care.
 As David Parkins, the ex-president of the College of Optometrists, emphasizes, the solution lies in increased integration and streamlined communication between primary and secondary eye care services. Implementing integrated digital platforms for referrals and feedback can enhance collaboration, improve patient outcomes, and reduce the burden on hospitals.
As David Parkins, the ex-president of the College of Optometrists, emphasizes, the solution lies in increased integration and streamlined communication between primary and secondary eye care services. Implementing integrated digital platforms for referrals and feedback can enhance collaboration, improve patient outcomes, and reduce the burden on hospitals.Leveraging optometrists’ expertise through shared care programs and direct digital referral pathways can alleviate the strain on eye hospitals and ensure timely access to care for patients with eye conditions.
-
Referral to Ophthalmology: Poor communication/lack of feedback
A recent study published in Ophthalmic and Physiological Optics revealed that in 73% of cases, the referring optometrist was unaware of the outcome of their referral.
This lack of closure can lead to unnecessary re-referrals, patient anxiety, and potential treatment delays that could result in preventable vision loss, especially considering the extended waiting times for hospital eye service appointments.
Effective referral in eye care requires a closed feedback loop, where referring providers receive timely updates and reports from specialists. However, studies have shown that up to 50% of primary care providers (PCPs) are unsure whether their patients have even been seen by the referred specialists. This disconnect necessitates time-consuming follow-up calls and manual data integration, increasing the risk of errors and jeopardizing patient care.
The absence of consistent feedback also impacts optometrists’ professional development. Without knowing the accuracy of their referrals, optometrists cannot identify areas for improvement or refine their diagnostic skills. This is particularly relevant for newly qualified practitioners who may benefit from feedback to enhance their clinical judgment.
Implementing electronic referral systems that include feedback mechanisms can significantly improve communication and close the feedback loop. This would enable optometrists to track the progress of their referrals, receive timely updates on patient outcomes, and make informed decisions about future referrals.
Technology is also bridging the gap in specialist communication by enabling secure online consultations, such as live chat with dedicated ophthalmologists. A notable example in the UK is Pocket Eye, a platform designed to empower eye care professionals with clinical advice, diagnostic and image support, and AI-powered OCT analysis.

Summing up
Implementing digital platforms that foster collaboration between eye care providers, increasing confidence in complex cases, and utilizing AI technologies to expedite diagnostics is crucial in a world where an aging population will increasingly rely on healthcare. Referral to ophthalmology from optometry should be effective, fast, and painless to eye care specialists and patients.
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes, not for clinical diagnosis purposes.
-
-

OCT Reports: Enhancing Diagnostic Accuracy
 Maria Martynova
07.06. 20238 min read
Maria Martynova
07.06. 20238 min readThe average OCT device is a significant investment, costing upwards of $40,000. As eye care specialists, we recognize the revolutionary power of OCT. However, patients often receive only a standard OCT report from this investment. Unfortunately, many patients are unaware of OCT’s true value and may not even know what it is. This raises a crucial question: are these standard reports truly reflecting the full diagnostic potential of such an expensive and sophisticated device? Are we, as professionals, maximizing the capabilities of this technology to ensure optimal patient care?
This article explores how OCT Reports address these shortcomings, enhancing diagnostic accuracy, treatment monitoring, referral efficiency, patient education, and audit readiness.
Common OCT reports and their limitations
Disclaimer: USA FDA 510(k) Class II; Altris Image Management System (Altris IMS); AI/ML models and components intended to use for research purposes, not for clinical diagnosis purposes.
How does the standard report look?

OCT has become a golden standard for diagnosing and monitoring many ocular pathologies, thanks to its unparalleled level of detail in ophthalmic imaging.
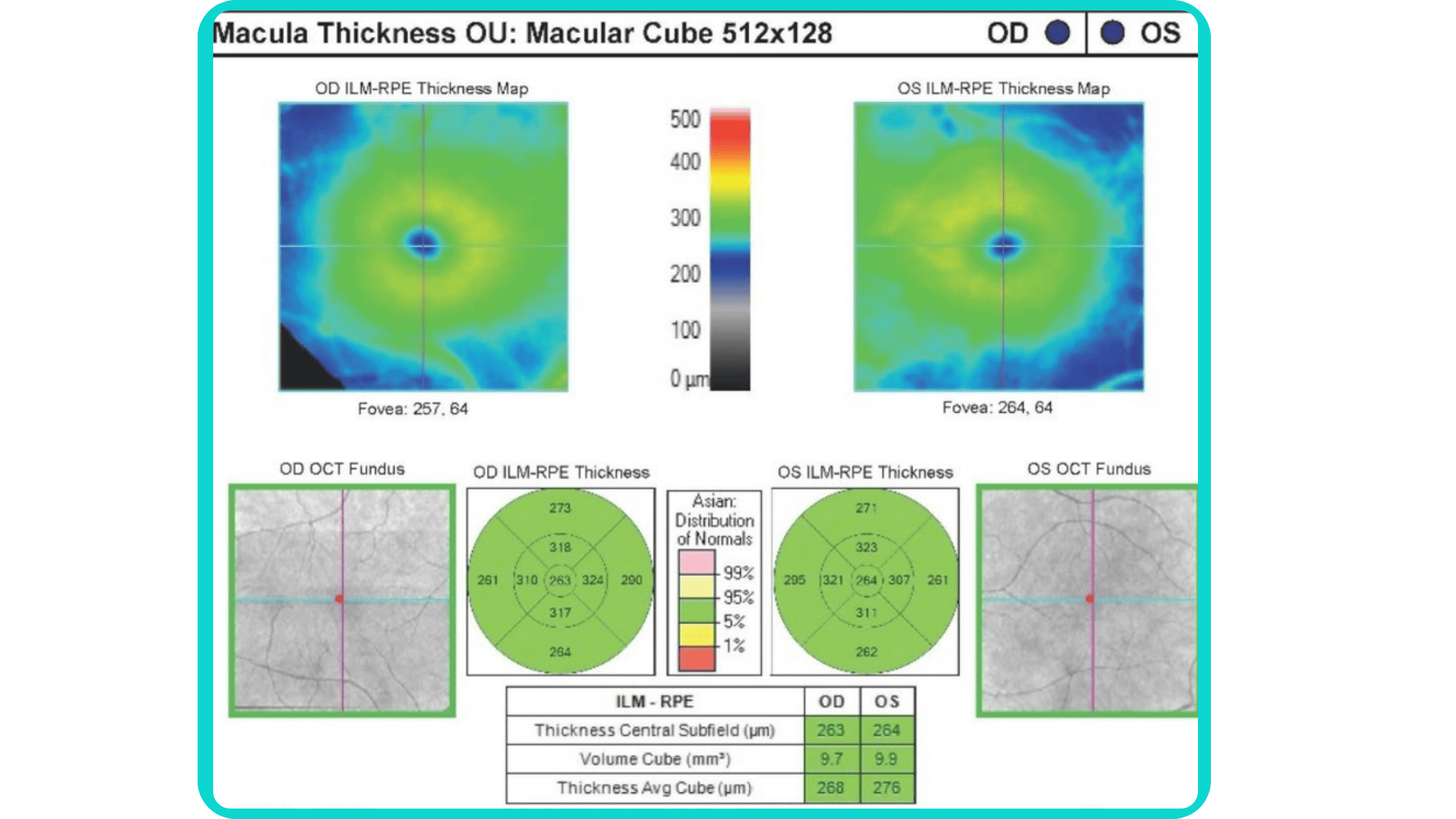
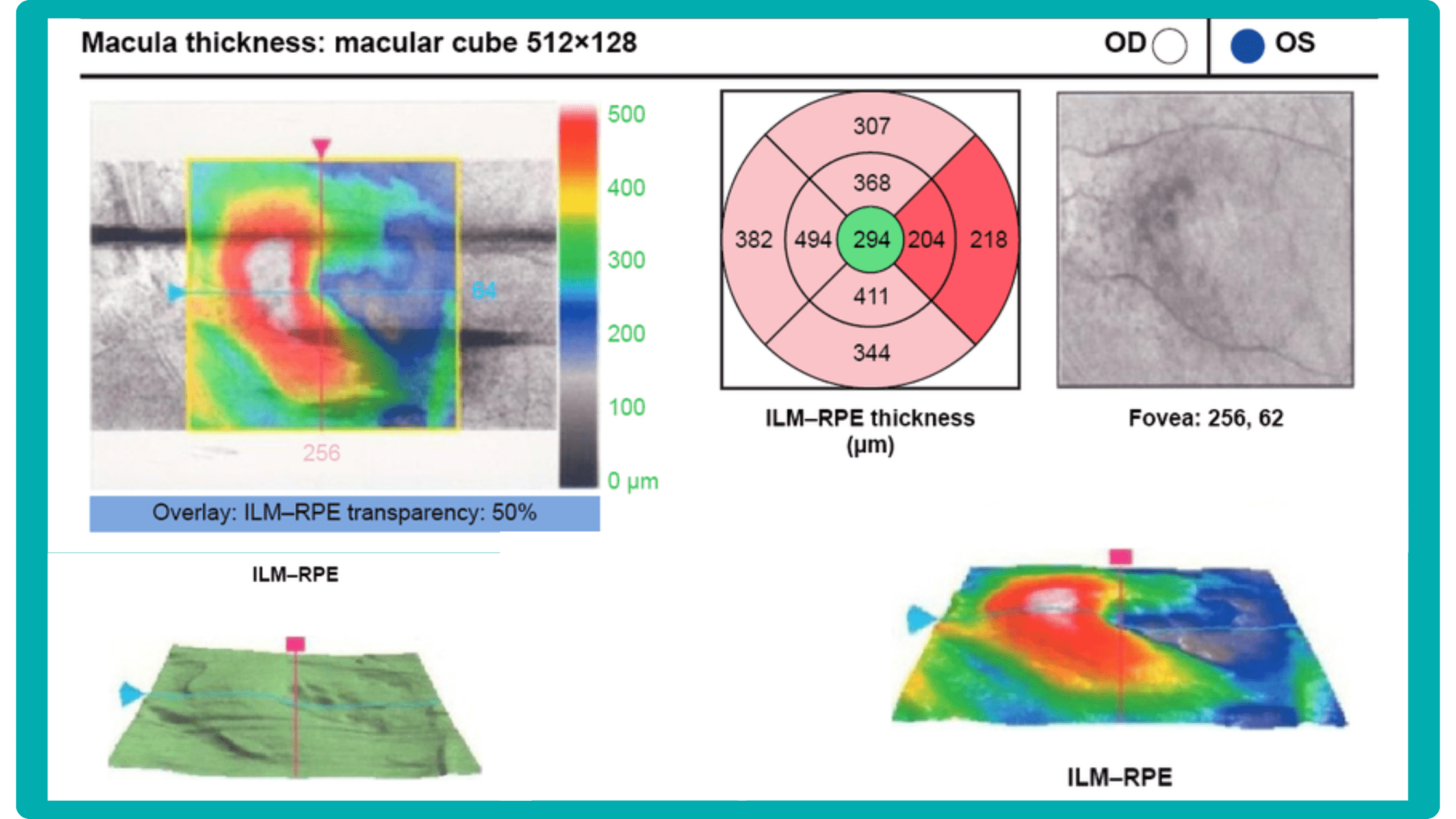
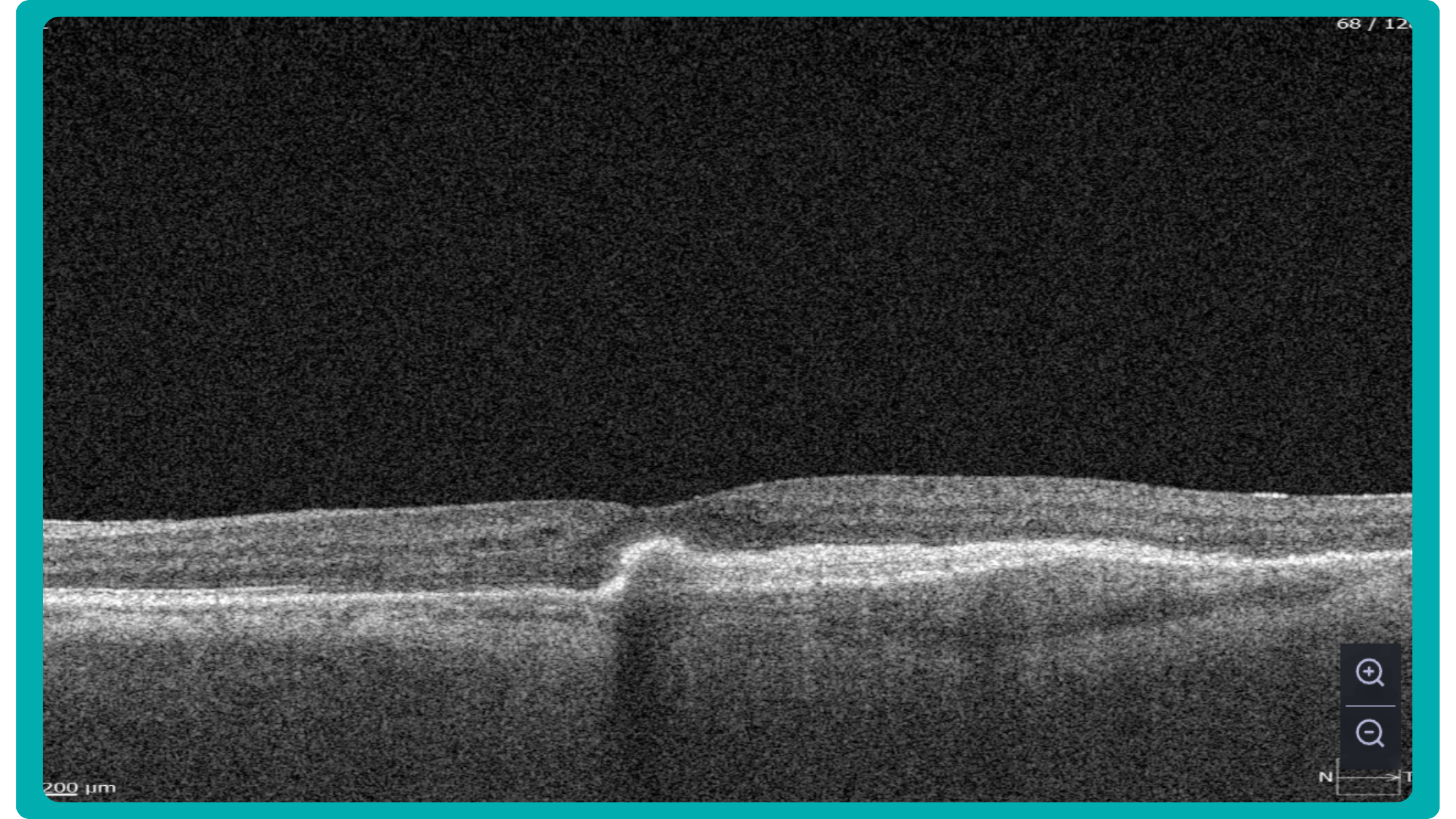
While retinal reports vary among OCT models, they typically include:- a foveally centered B-scan,
- a quantitative thickness map,
- and a semi-quantitative thickness map.
The B-scan offers a visual snapshot of foveal architecture and confirms proper scan centering. The quantitative thickness map employs the ETDRS sector map to measure retinal thickness within a 6mm circle around the fovea, with specific measurements for the foveal sector (1mm), inner macular ring (3mm), and outer macular ring (6mm).
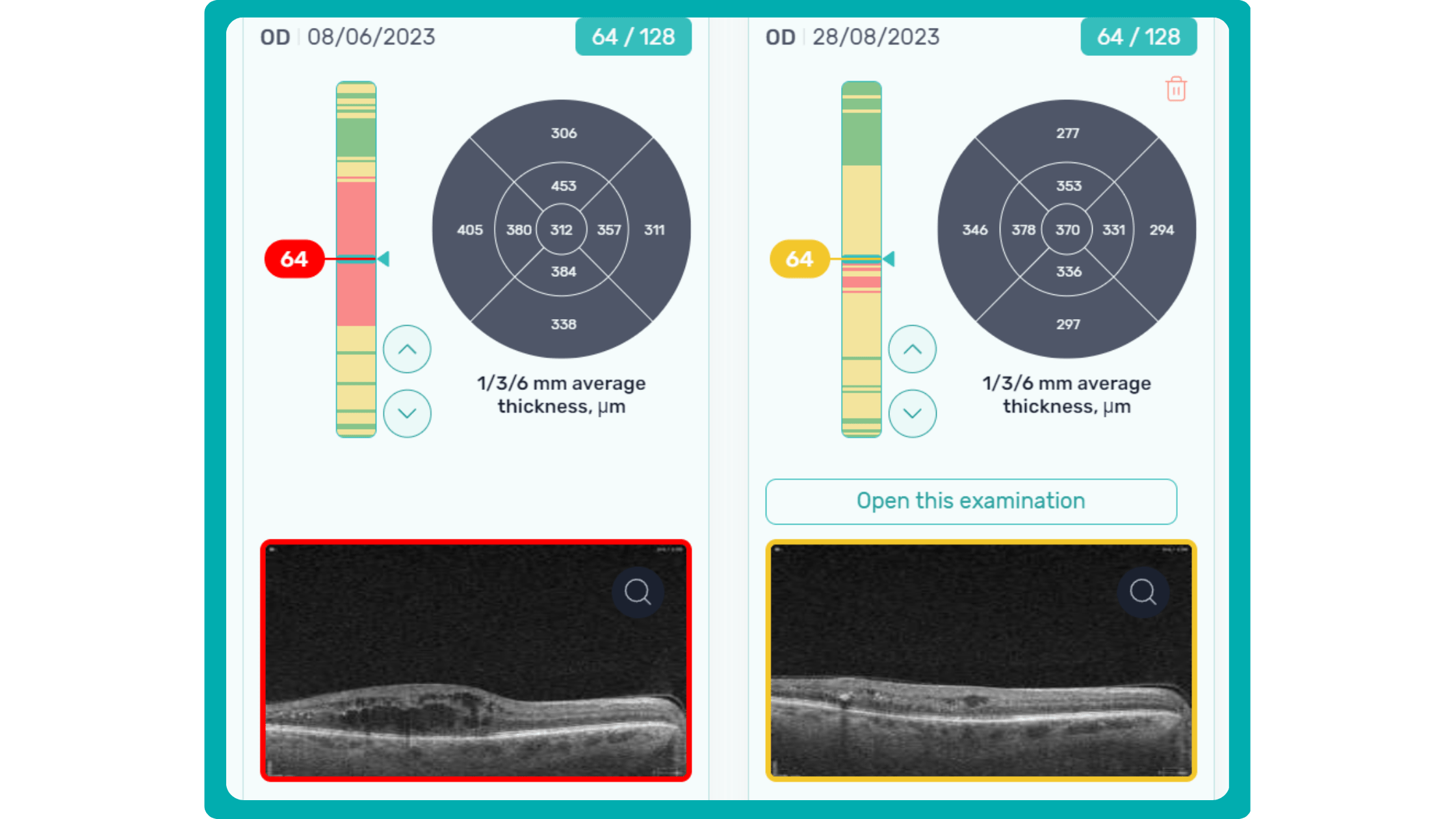
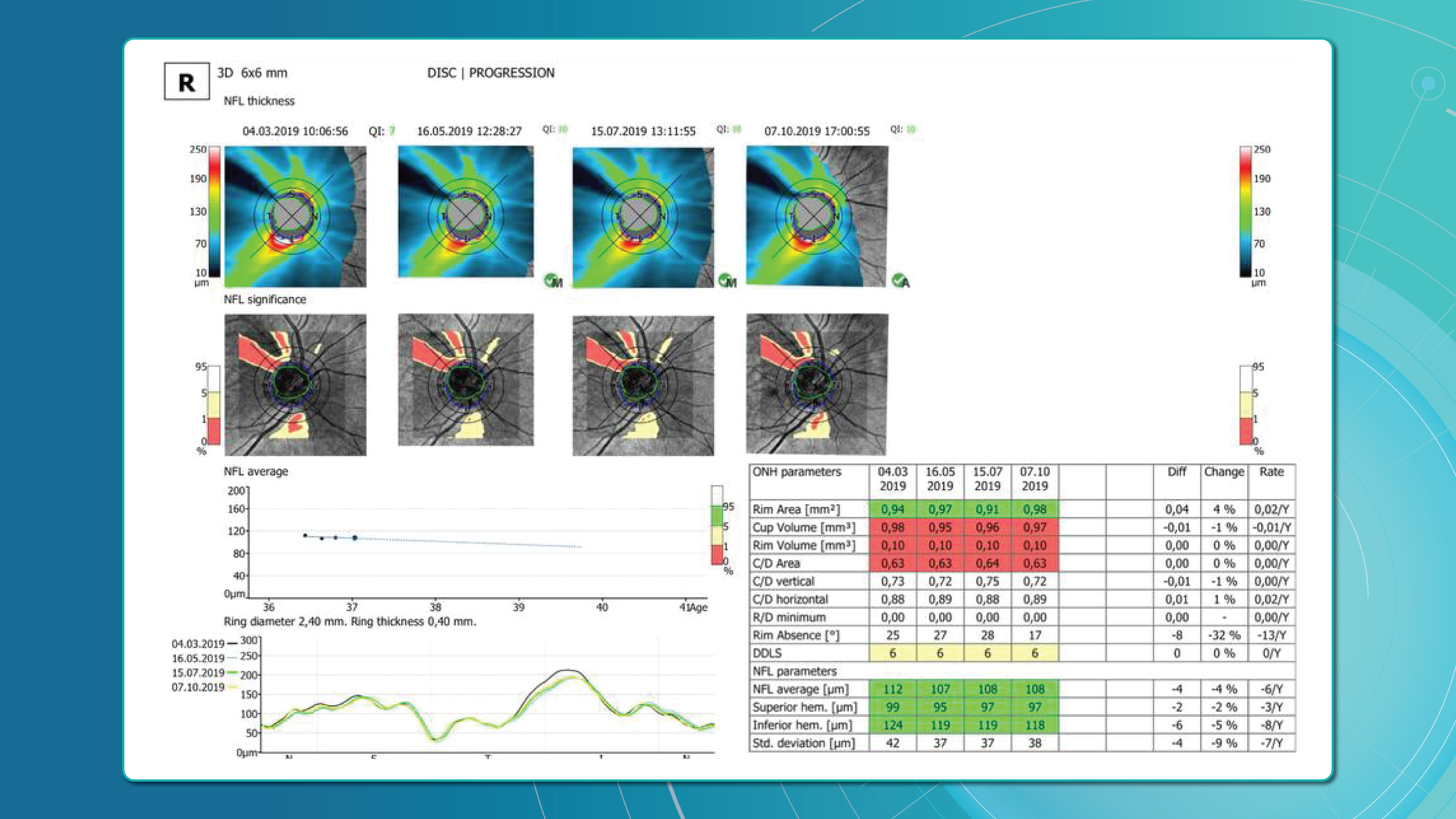
Progression analytics enable comparison of serial macular scans, which is invaluable for managing vitreomacular interface disorders and macular edema. The semi-quantitative thickness map provides a broader overview of retinal thickness throughout the scan.
Given this amount of data, it is challenging to identify subtle and localized retinal pathological changes. As a result, entire OCT datasets are represented by few aggregated values, and the standard OCT reports generated by most devices often rely on significant data reduction to simplify interpretation, which you can usually not customize.
OCT report interpretation: 3 methods exist for displaying OCT data
Firstly, acquired 2D image slices are presented individually. This allows for detailed examination, but navigating through numerous images can be cumbersome, particularly with large datasets.

Secondly, a fundus image is displayed with superimposed retinal layers. This facilitates linking layers to the fundus, but only one layer can be examined at a time, hindering the analysis of multiple layers simultaneously.

Thirdly, the OCT tomogram is visualized in 3D, providing a comprehensive overview, but adjusting the visual representation often has limitations. Additionally, combined 3D visualizations of the tomogram and layers are typically unavailable, potentially obscuring spatial relationships.

While existing reports offer diverse approaches to managing, analyzing, and presenting OCT data, each solution focuses on specific aspects and lacks customization. The situation becomes even more complex if scans come from different OCT devices, as manufacturers only provide software for the data for proprietary OCT scanners. Consequently, no approved way of viewing, analyzing, or comparing data from different manufacturers exists.
Furthermore, there are limited possibilities for implementing prototypes to perform such tasks since software libraries are provided with exclusive licenses and incomplete data specifications. Hence, managing and analyzing OCT data and relating them to other information are challenging and time-consuming tasks.
Often, supplementary software is utilized to overcome these limitations by providing additional information, visualizing and emphasizing data differently, and enabling the selection of relevant subsets.
How can customized reports for OCT help?

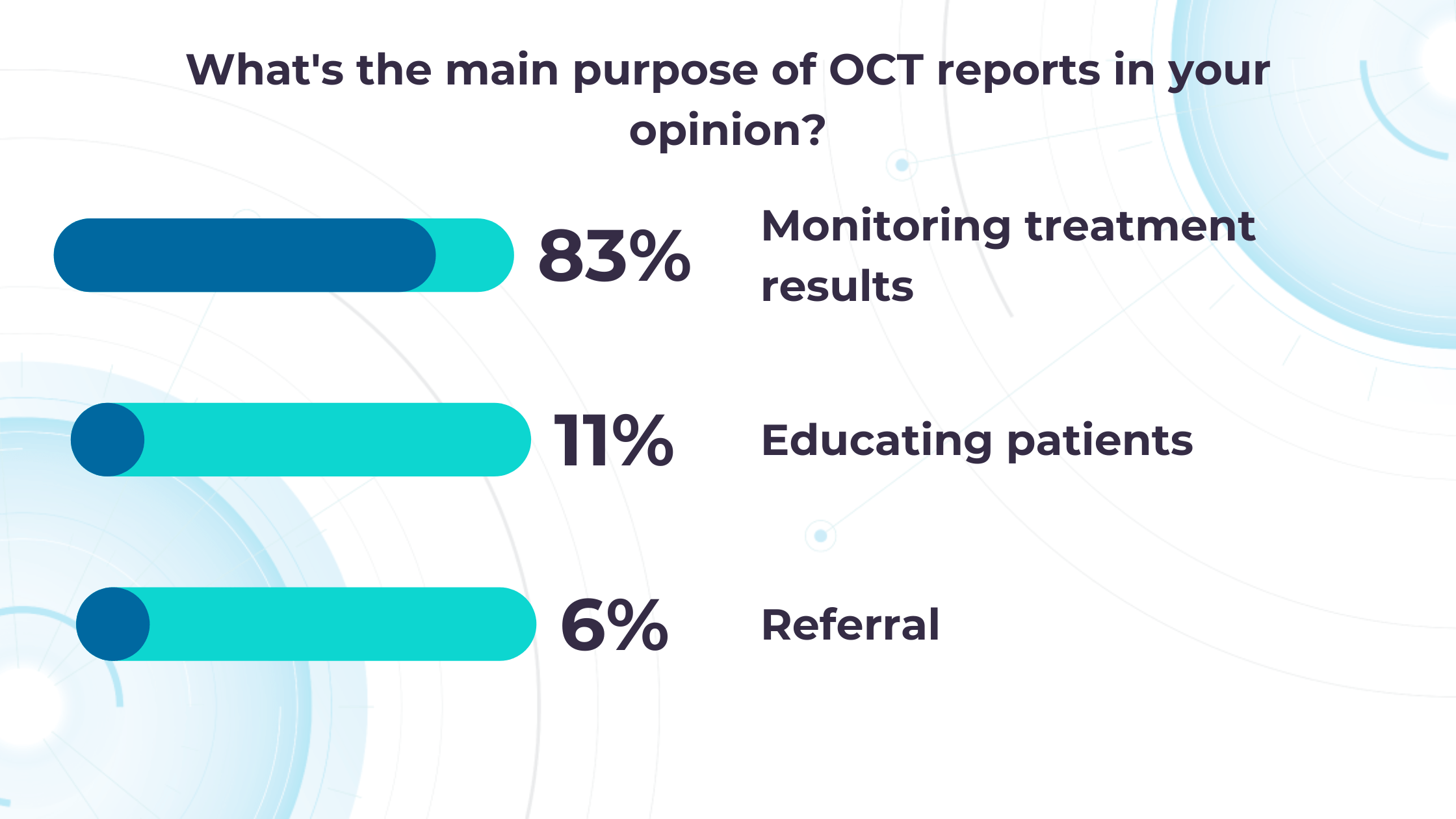
Altris AI’s recent survey has revealed that the key benefits of OCT technology for eye care specialists lie in treatment monitoring, patient education, and referral optimization.

-
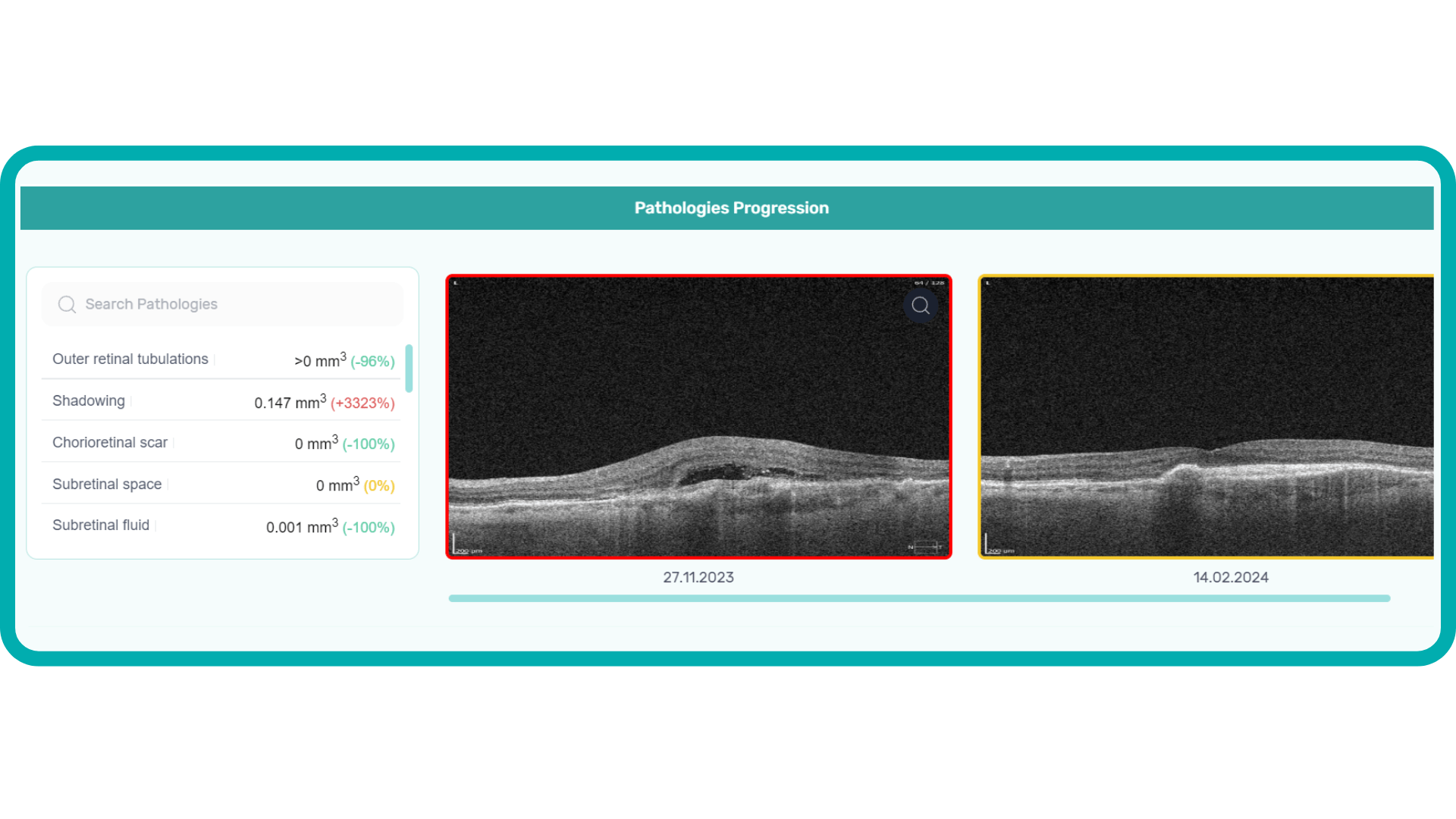
Measuring treatment progress: biomarkers tracking, pathology progression
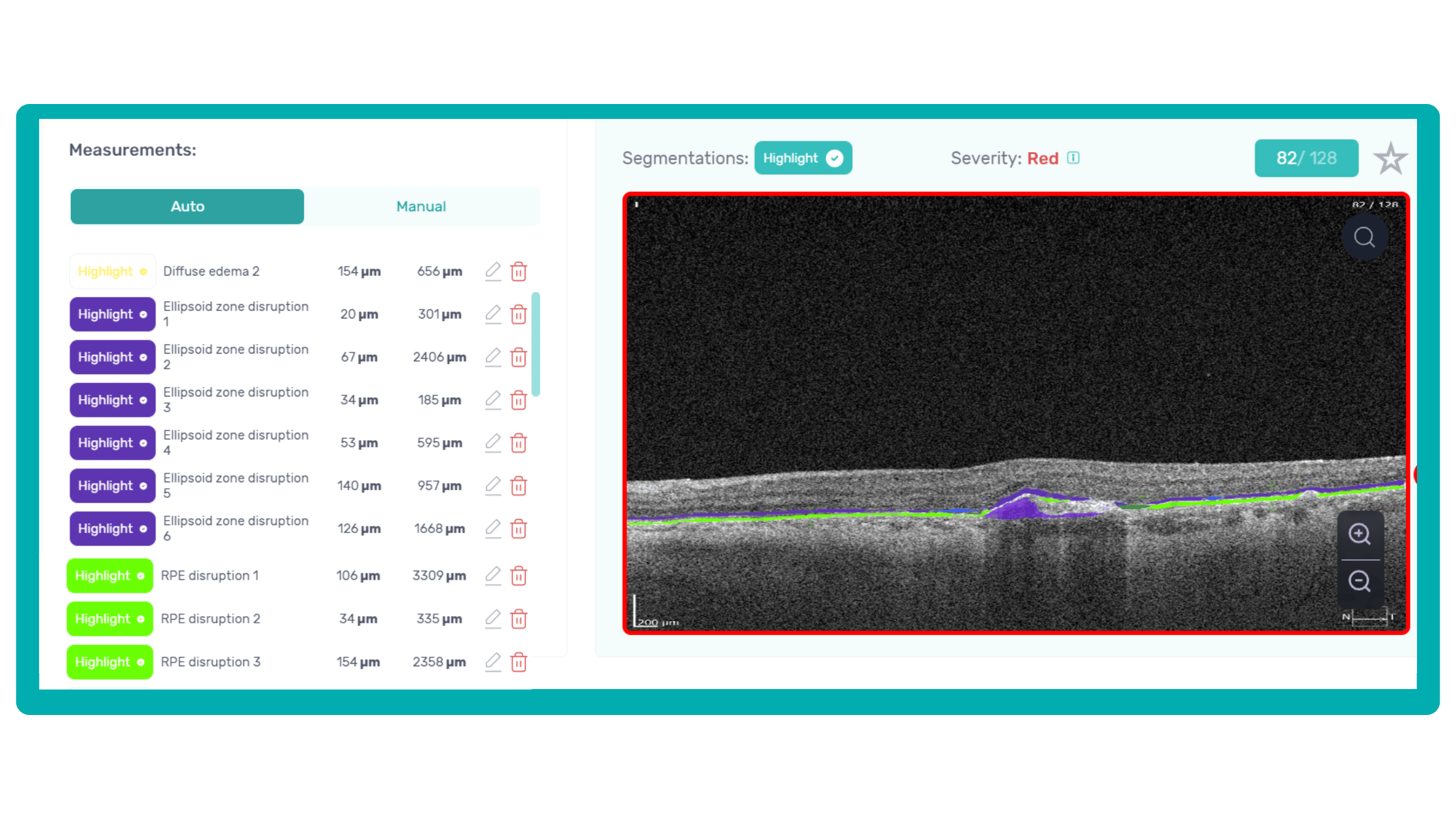
Imaging biomarkers are a particularly attractive option for clinical practice due to their non-invasive and real-time nature. Quantitative measurements of retinal thickness, fluid volume, and other biomarkers relevant to diseases like diabetic retinopathy and age-related macular degeneration aid in treatment monitoring.

OCT reports with customized measurements and selected biomarkers, retinal layers, or segments allow for precise focus on treatment monitoring and patient response to therapy. This personalized approach enhances clinical decision-making by highlighting each case’s most relevant information.

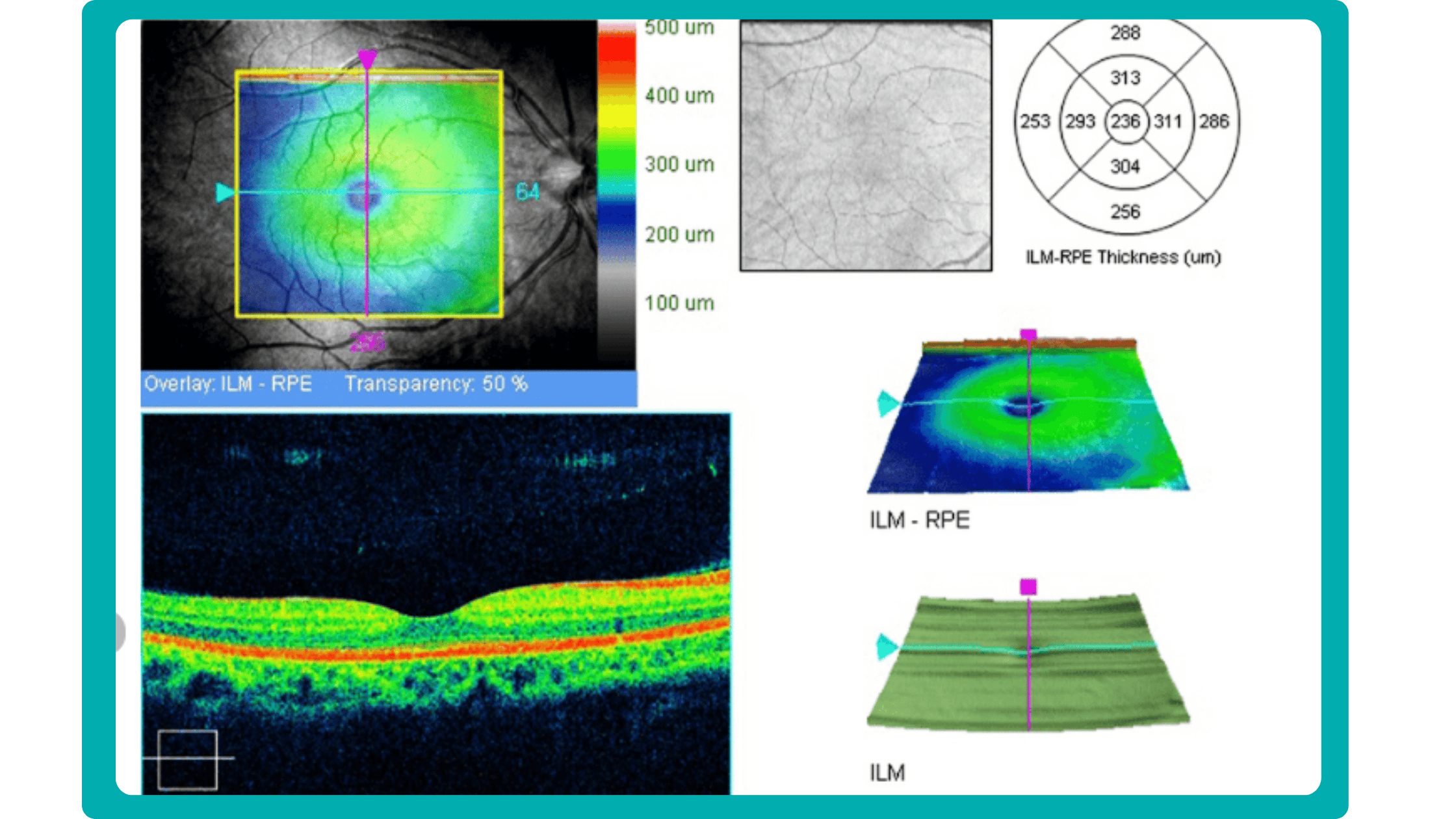
In current clinical practice, macular damage assessment typically involves measuring the distance between the ILM and RPE layers, summarized in a post-scan report.

However, these reports often fall short of visualization best practices, employing ineffective or inconsistent color schemes. Additionally, they lack flexibility, with static visuals preventing in-depth examination of specific details. Despite these limitations, these reports remain valuable for many clinicians by distilling complex data into a manageable format.
Enhanced OCT data visualization offers a promising solution to these challenges. It enhances report clarity and comprehensibility while preserving the richness of the underlying data.
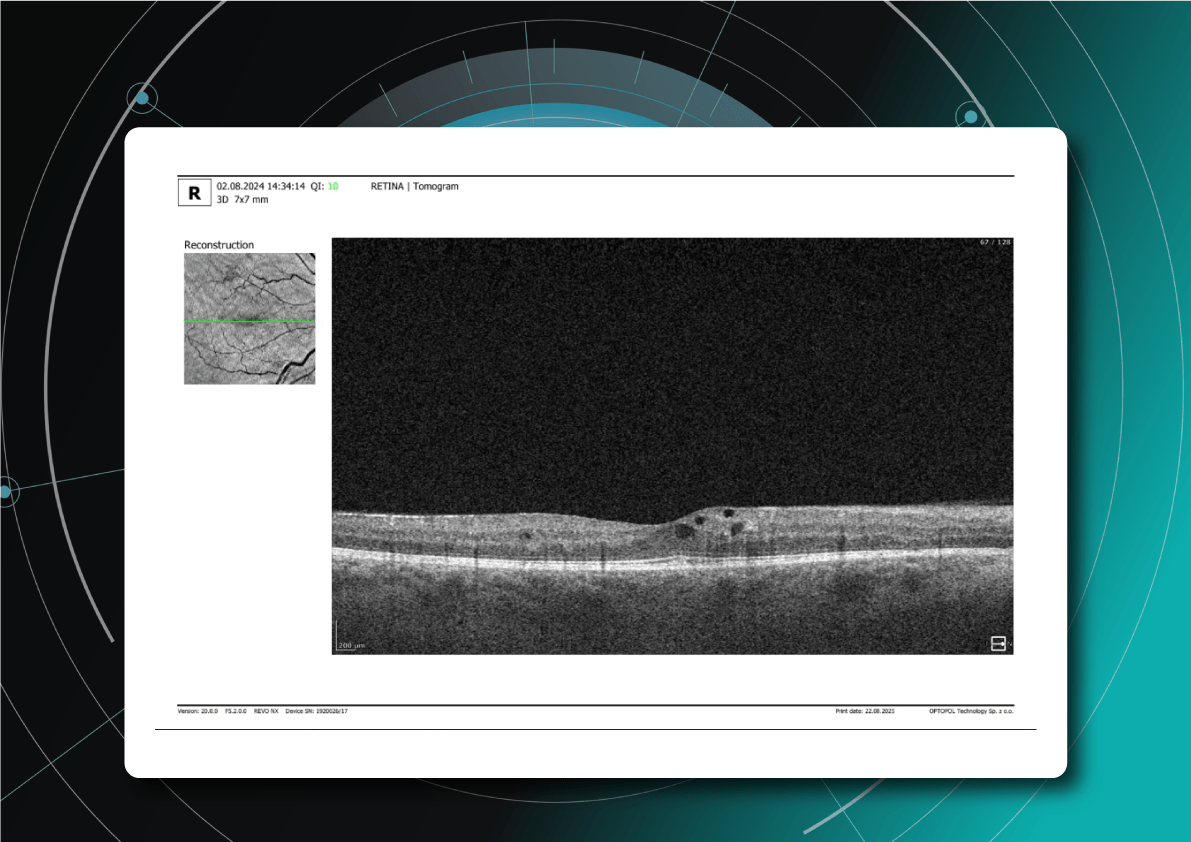
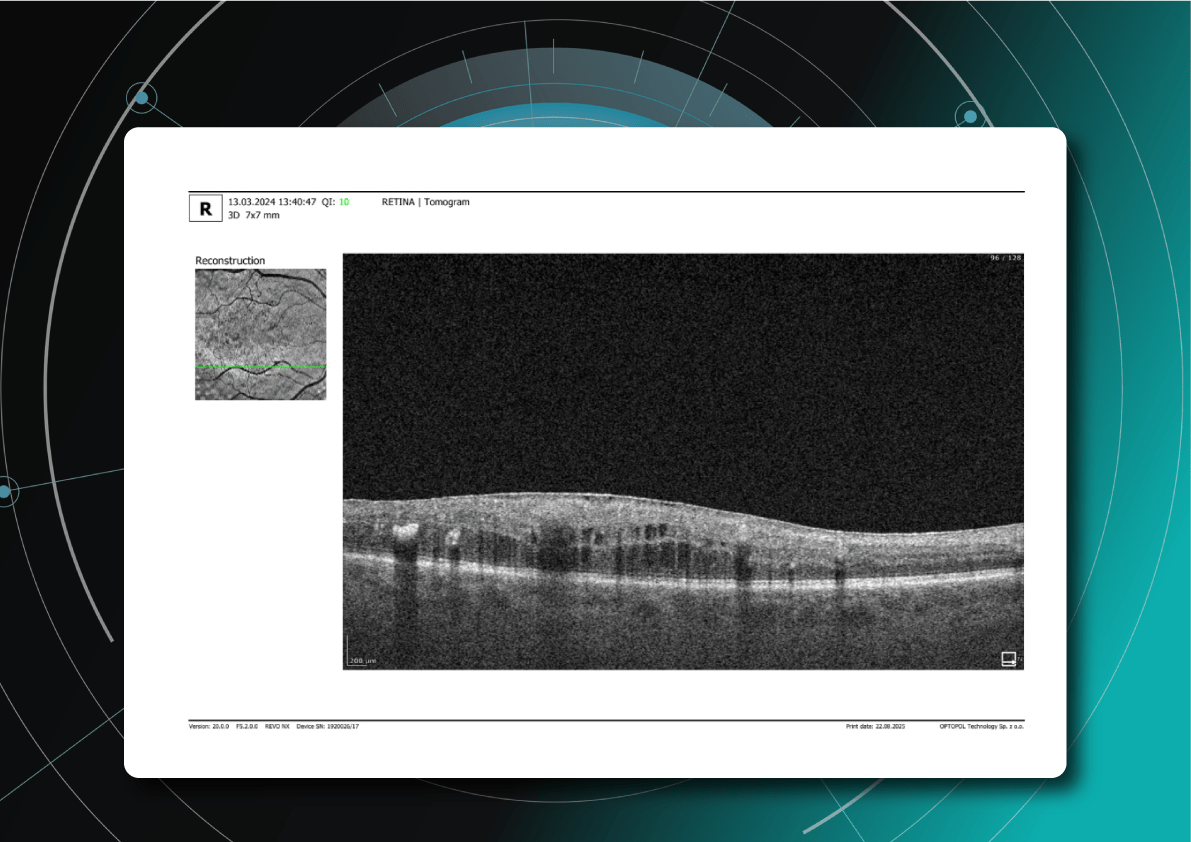
Let’s explore how this applies to a clinical case, such as monitoring a patient with Wet AMD during follow-up visits.

Data demonstrates that OCT findings can reveal the onset or progression of neovascular AMD before a patient reports new symptoms or changes in visual acuity. In fact, OCT images are reported to have the best diagnostic accuracy in monitoring nAMD disease states. This underscores the importance of key OCT findings or biomarkers in personalizing anti-VEGF treatment, achieving disease control, and reducing monitoring burdens.

Central Retinal Thickness emerged as one of the earliest OCT biomarkers used as an outcome measure in clinical trials for nAMD.
However, due to confounding factors, CRT’s use in outcome-based assessments of nAMD varies. Thus, it is essential to evaluate additional morphological changes alongside retinal thickness and their relationships with functional outcomes.
It has been reported that OCT images have the best diagnostic accuracy in monitoring nAMD disease states.
Another finding that is correlated with a worsening VA due to the associated photoreceptor defects is any damage to the four outer retina layers, including the RPE, interdigitation zone (IZ), ellipsoid zone (EZ), and external limiting membrane band (ELM).

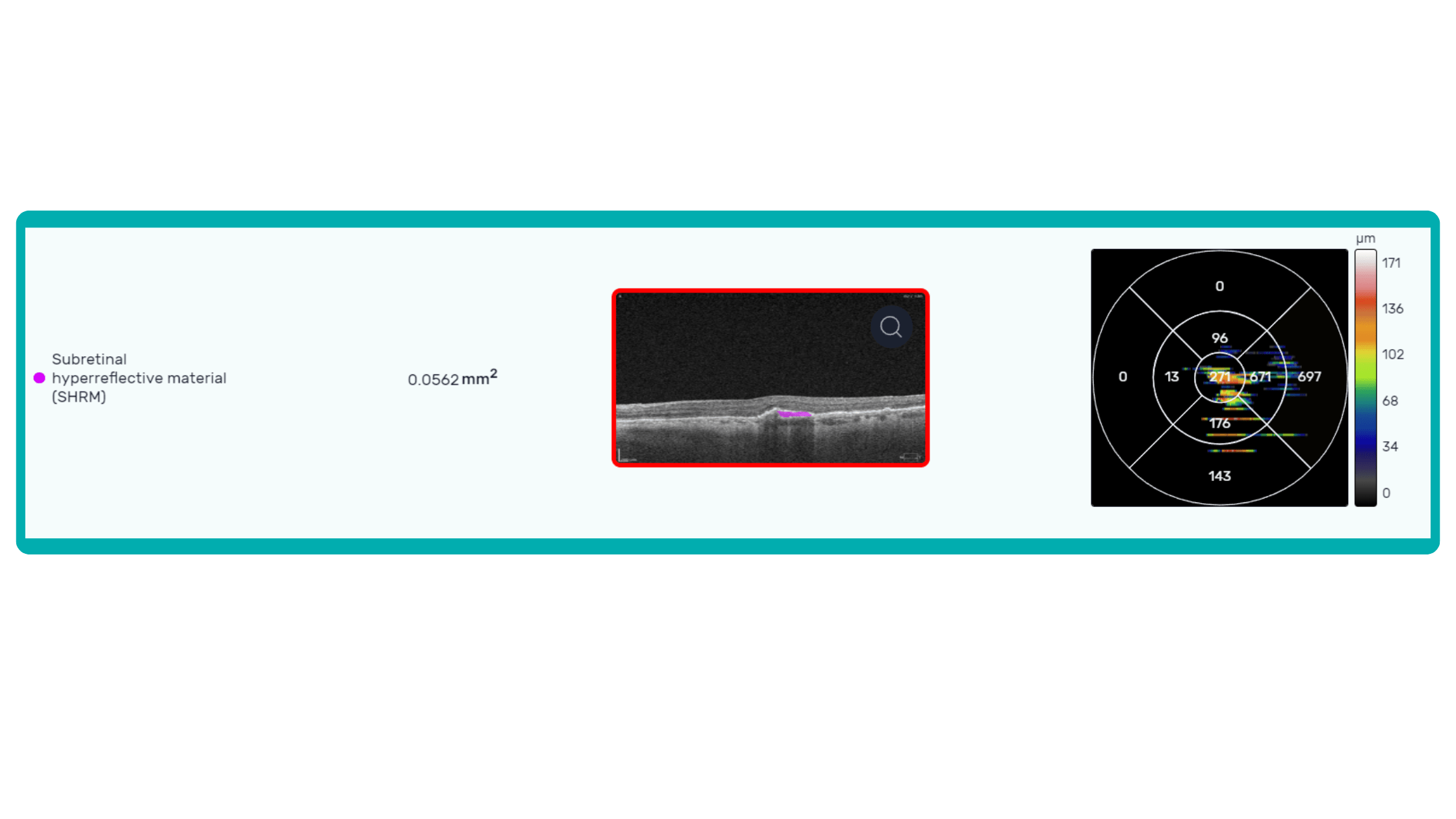
OCT is a valuable imaging tool for visualizing subretinal hyperreflective material (SHRM). It can automatically identify and quantify SHRM and fluid and pigment epithelial detachment to calculate the overall risk of worsening visual outcomes associated with SHRM.

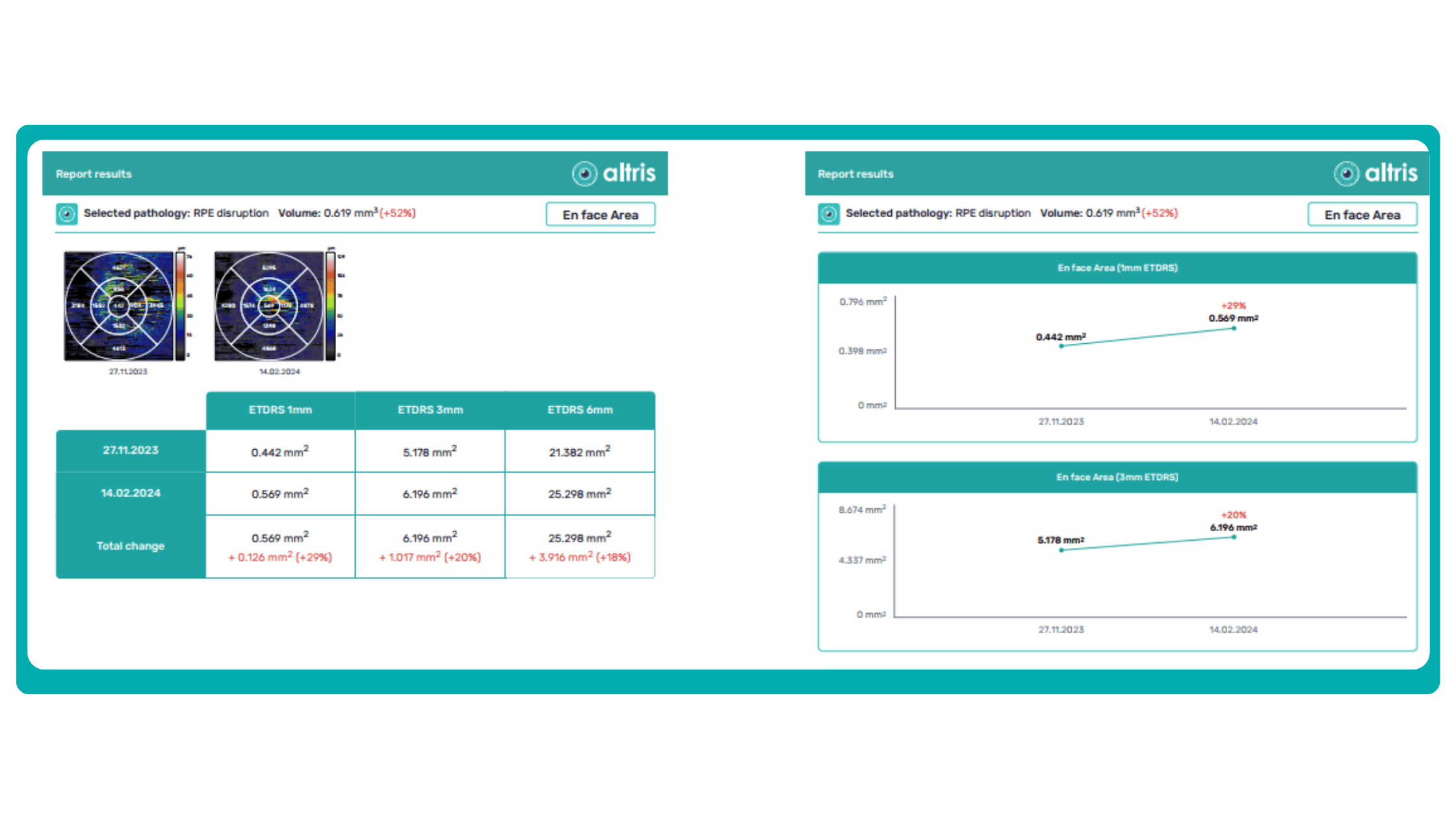
Subsequent follow-up visits will then display the most relevant picture, highlighting the most pertinent biomarkers for tracking a particular pathology (wet AMD in our example) and comparing their volume, progression, or regression through visits.

Another helpful option is retinal layer segmentation, which focuses solely on the retinal layers of interest for the specific case.
This level of customization empowers clinicians with a comprehensive yet targeted view of the patient’s condition. It saves time from manually detecting anomalies on scans and facilitates informed decision-making and personalized treatment plans.
-
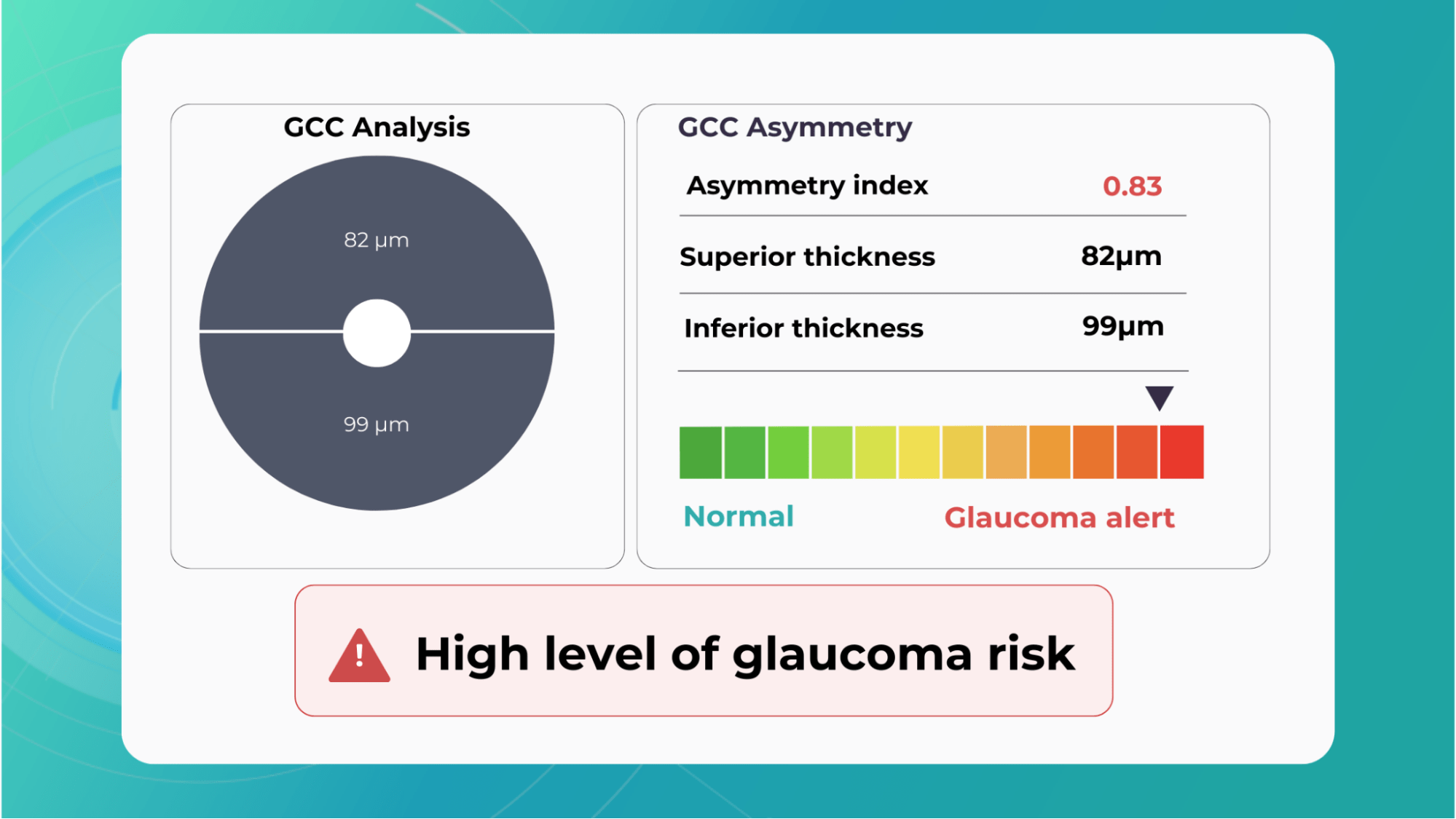
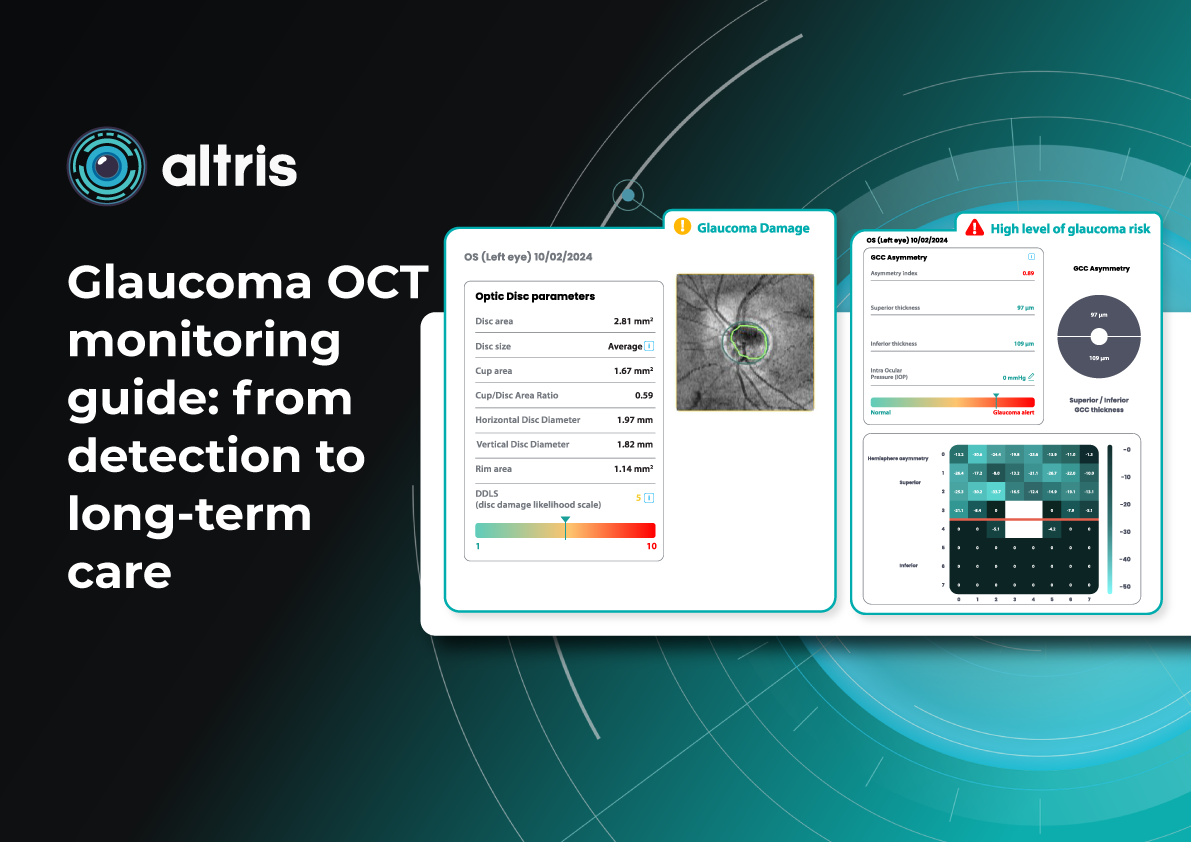
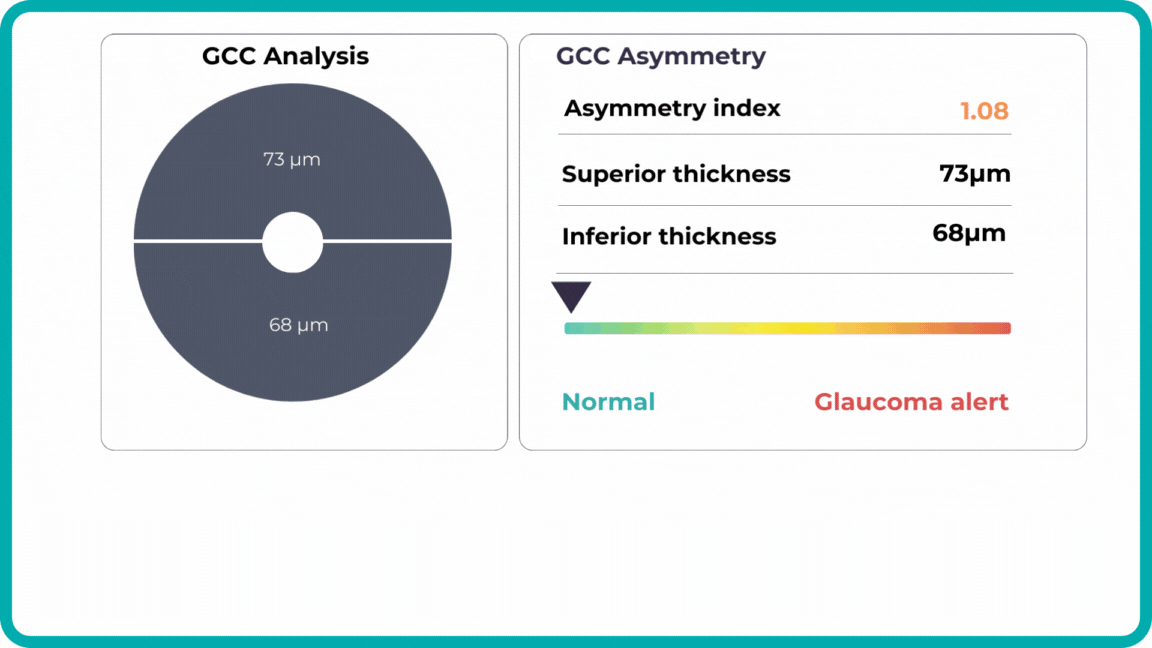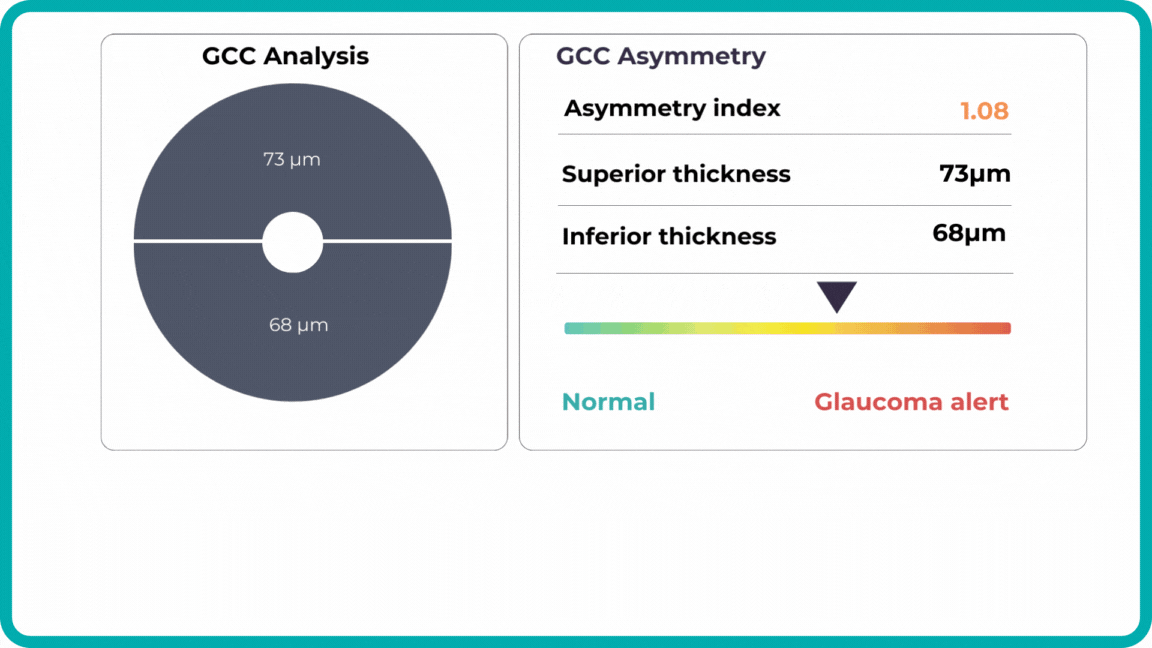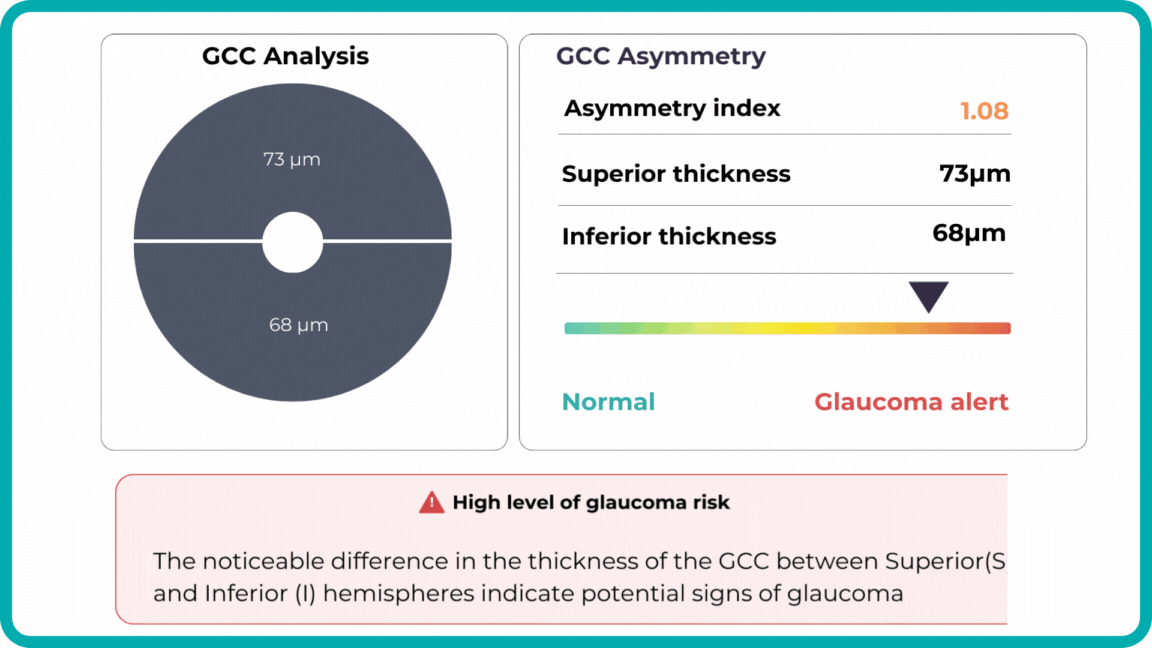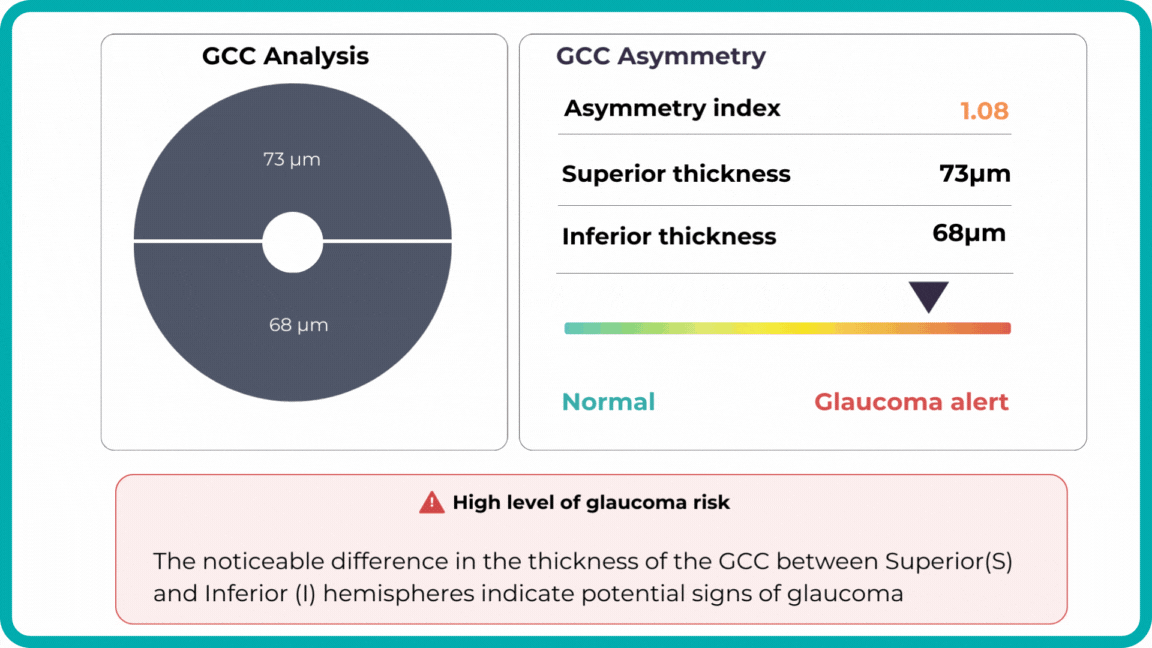
Glaucoma risk evaluation
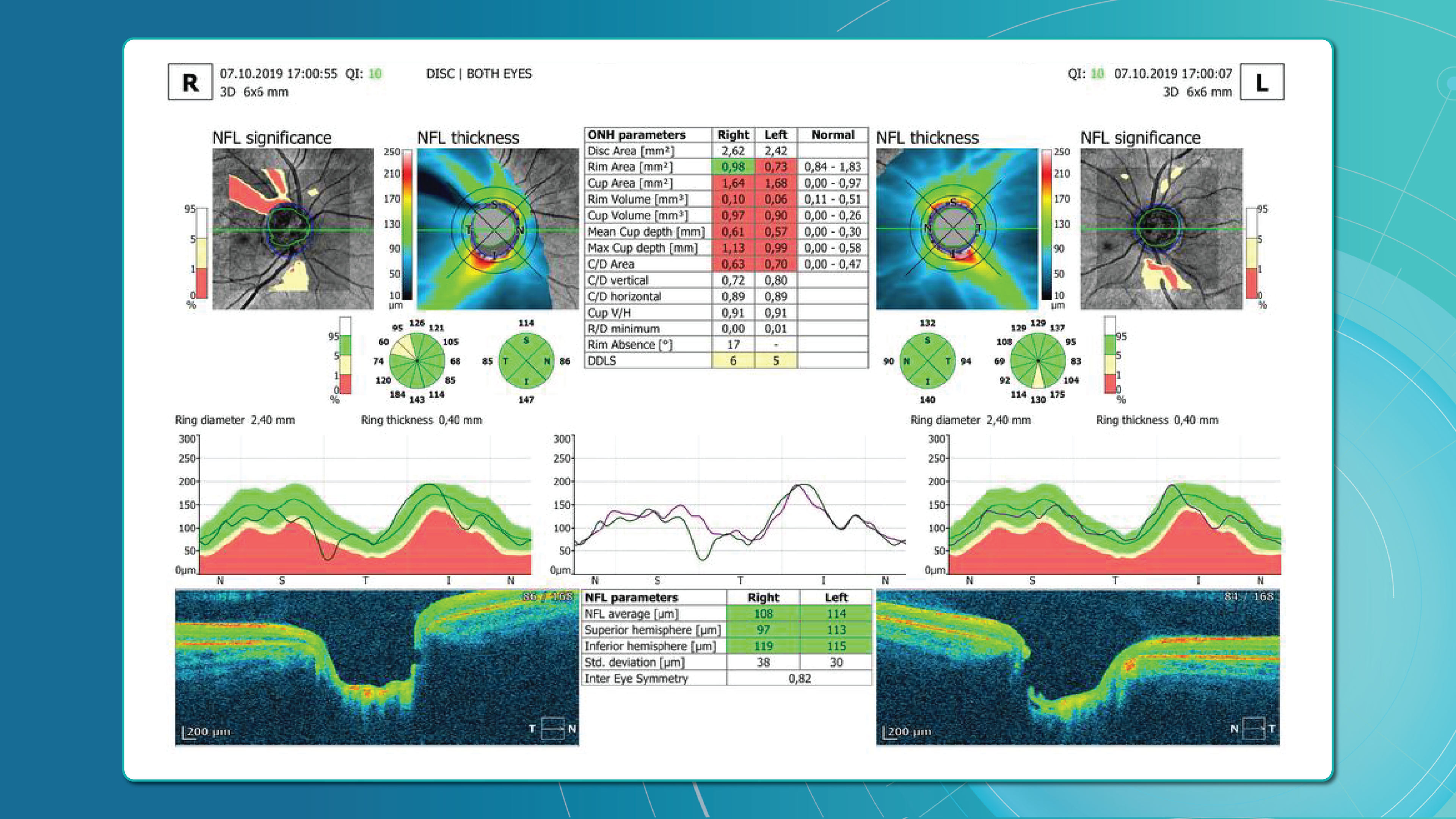
Millions risk irreversible vision loss due to undiagnosed glaucoma, underscoring the need for improved early detection. Current tests often rely on observing changes over time, delaying treatment assessment and hindering early identification of rapid disease progression. OCT frequently detects microscopic damage to ganglion cells and thinning across these layers before changes are noticeable through other tests. However, the earliest signs on the scan can still be invisible to the human eye.
AI algorithms offer insights into glaucoma detection by routinely analyzing the ganglion cell complex, measuring its thickness, and identifying any thinning or asymmetry to determine a patient’s glaucoma risk without additional clinician effort.

Another significant benefit of AI systems is that OCT for glaucoma usually utilizes a normative database to assess retinal normality. However, these databases are limited in size and represent an average of a select group of people, potentially missing early glaucoma development in those who deviate from the “norm.” Conversely, individuals may be unnecessarily referred for treatment due to not fitting the “normal” profile, even if their eyes are healthy.
-
Crafting effective referral
In the UK, optometrists are crucial in initiating referrals to hospital eye services (HES), with 72% originating from primary care optometric examinations. While optometrists generally demonstrate proficiency in identifying conditions like cataracts and glaucoma, discrepancies in referral thresholds and unfamiliarity with less common pathologies can lead to unnecessary or delayed referrals.

At the same time, an evaluation of incoming letters from optometrists in a glaucoma service found that 43% of the letters were considered “failures” because they did not convey the necessity and urgency of the referral.
So, having an elaborate record of the entire clinical examination in addition to a referral letter is crucial.

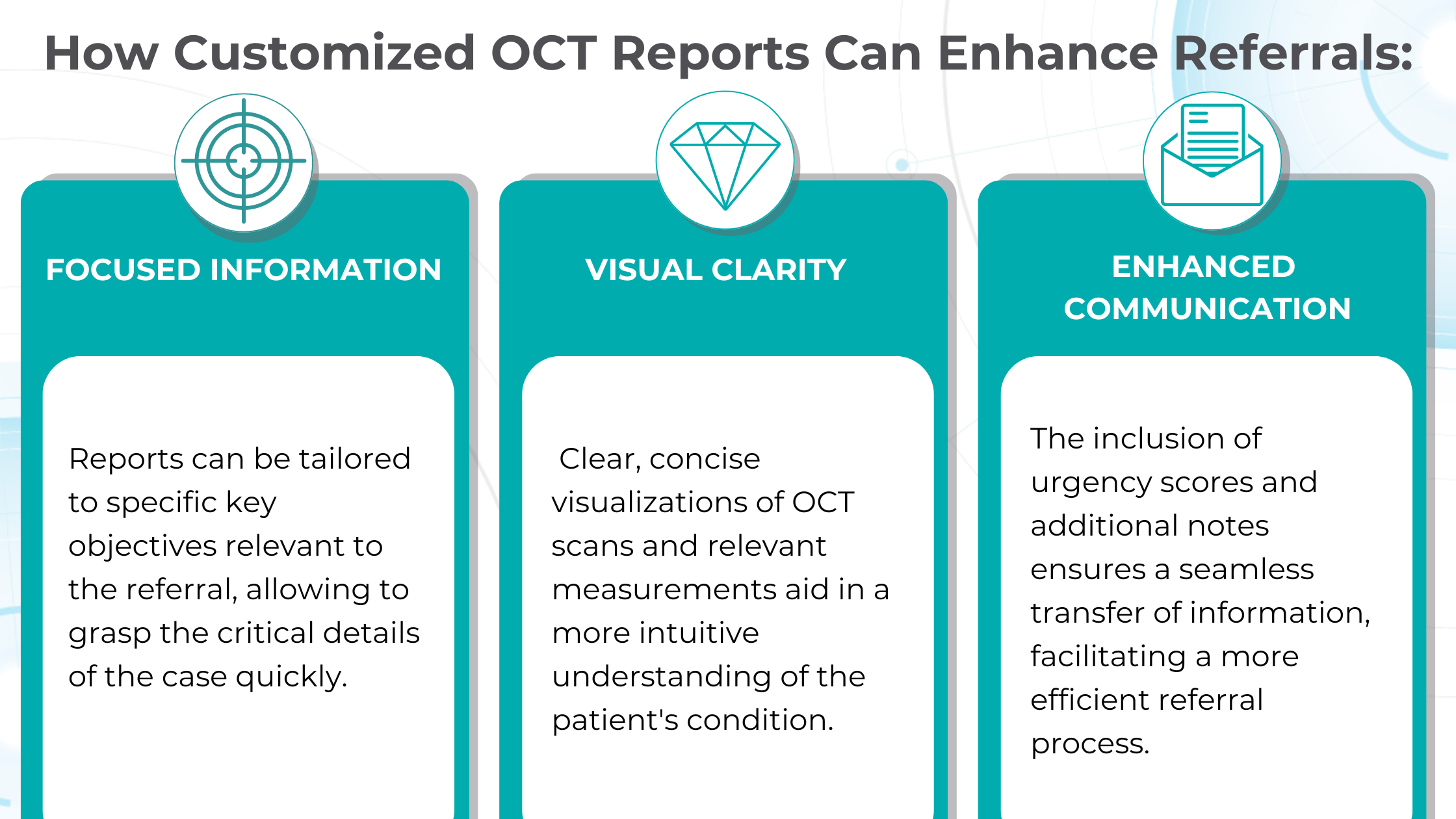
Customized OCT reports solve this challenge by streamlining the referral process and improving communication between optometrists and ophthalmologists. These reports can significantly reduce delays and ensure patients receive timely care by providing comprehensive and relevant information upfront.
-
Patient Education

Patient education and involvement in decision-making are vital for every medical field and crucial for ophthalmology, where insufficient patient engagement can lead to irreversible blindness.

Research specifically targeting the ophthalmology patient population, which often includes older and potentially visually impaired individuals, reveals a clear preference for materials their eye care provider endorsed.

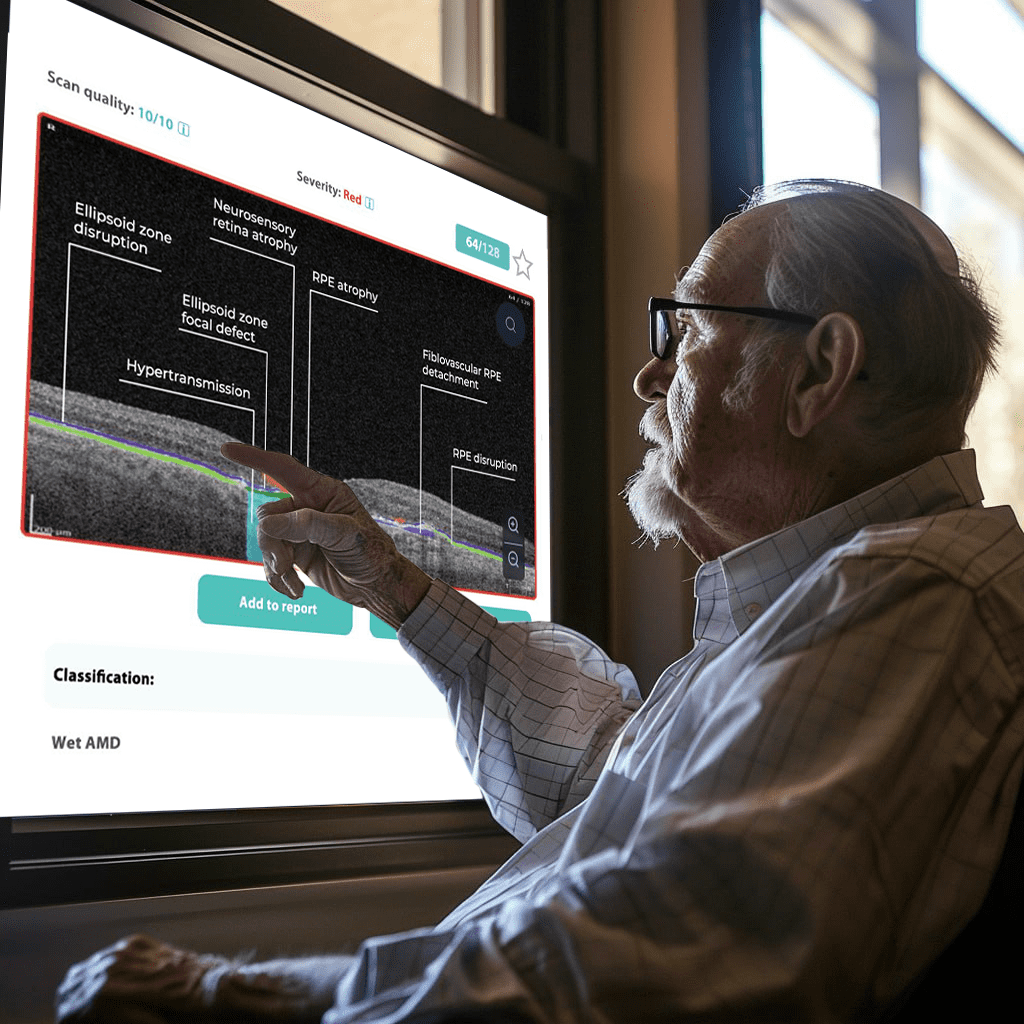
Providing explicit visual representations of diagnoses can significantly improve patient understanding and compliance. Seeing photos of their condition, like glaucoma progression, builds trust and reinforces the importance of treatment recommendations.
Surveying eye care professionals specializing in dry eye disease revealed a strong emphasis on visual aids during patient education.
Photodocumentation is a favored tool for demonstrating the condition to asymptomatic patients, tracking progress, and highlighting treatment’s positive outcomes.
The visual approach provides tangible evidence of the benefits of their treatment investment, allowing for a deeper understanding of the “why” behind treatment recommendations and paving the way for ongoing collaboration with the patient.

Color-coded OCT reports for pathologies and their signs, severity grading, and pathology progression over time within its OCT analysis highlight the littlest bits that a patient’s unprepared eye would miss otherwise. With follow-up visits, patients can see what’s happening within their eyes and track the progress of any conditions during treatment.

-
Updating EMR and Audit readiness
OCT reports are crucial components of a patient’s medical history and are essential for accurate diagnosis, personalized treatment, and ongoing monitoring. The streamlined process of integrating OCT data into EMR ensures that every eye scan, with its corresponding measurements, biomarkers, and visualizations, becomes an easily accessible part of the patient’s medical history.
This is crucial for continuity of care and simplifies the audit process, providing a clear and comprehensive record of the patient’s eye health over time. Just optometry chains alone can perform an imposing volume of OCT scans, with some reaching upwards of 40,000 per week. While this demonstrates the widespread adoption of this valuable diagnostic tool, it also presents a challenge: the increased risk of missing subtle or early-stage pathologies amidst the sheer volume of data.
Enhanced OCT reports offer a solution by providing a crucial “second look” at scan results. While not foolproof, this double-check significantly reduces the risk of overlooking abnormalities, ultimately improving patient outcomes and safeguarding the clinic’s reputation.
In audits, comprehensive OCT reports are critical in ensuring regulatory compliance. As the Fundamentals of Ophthalmic Coding states, “It is the responsibility of each physician to document the interpretations as promptly as possible and then communicate the findings with the patient… to develop a fail-safe way to ensure that your interpretations are completed promptly.”
Auditors typically look for several key elements in OCT reports:
- Physician’s Order: Document the test order, indicating which eye(s) and the medical necessity.
- Interpretation and Report: The physician analyzes the scan results, including any identified abnormalities or concerns.
- Timely Completion: Prompt documentation and communication of findings to the patient.
Customisable OCT reports can streamline this process by generating comprehensive reports that meet these requirements. These reports include detailed measurements, biomarker analysis, and clear visualizations, making it easier for physicians to review, interpret, and document their findings efficiently.
Summing up
Standard OCT reports, while valuable, often need more customization due to data reduction and lack of customization. The inability to visualize multiple scans simultaneously or compare data from different devices hinders comprehensive analysis. Enhanced OCT reports address these limitations by offering detailed visualizations, customizable measurements, and biomarker tracking.
Customisable OCT reports aid in the early detection and monitoring of diseases like wet AMD and glaucoma, empowering clinicians with accurate diagnoses and personalized treatment plans. Additionally, they streamline referrals by providing focused information and clear visualizations, reducing delays and improving communication between optometrists and ophthalmologists.
These comprehensive reports also enhance patient education by offering clear visual representations of their conditions and treatment progress, fostering better understanding and compliance. Moreover, with detailed documentation and analysis, detailed reports ensure audit readiness for eye care professionals, mitigating the risk of missed pathologies and upholding regulatory compliance.











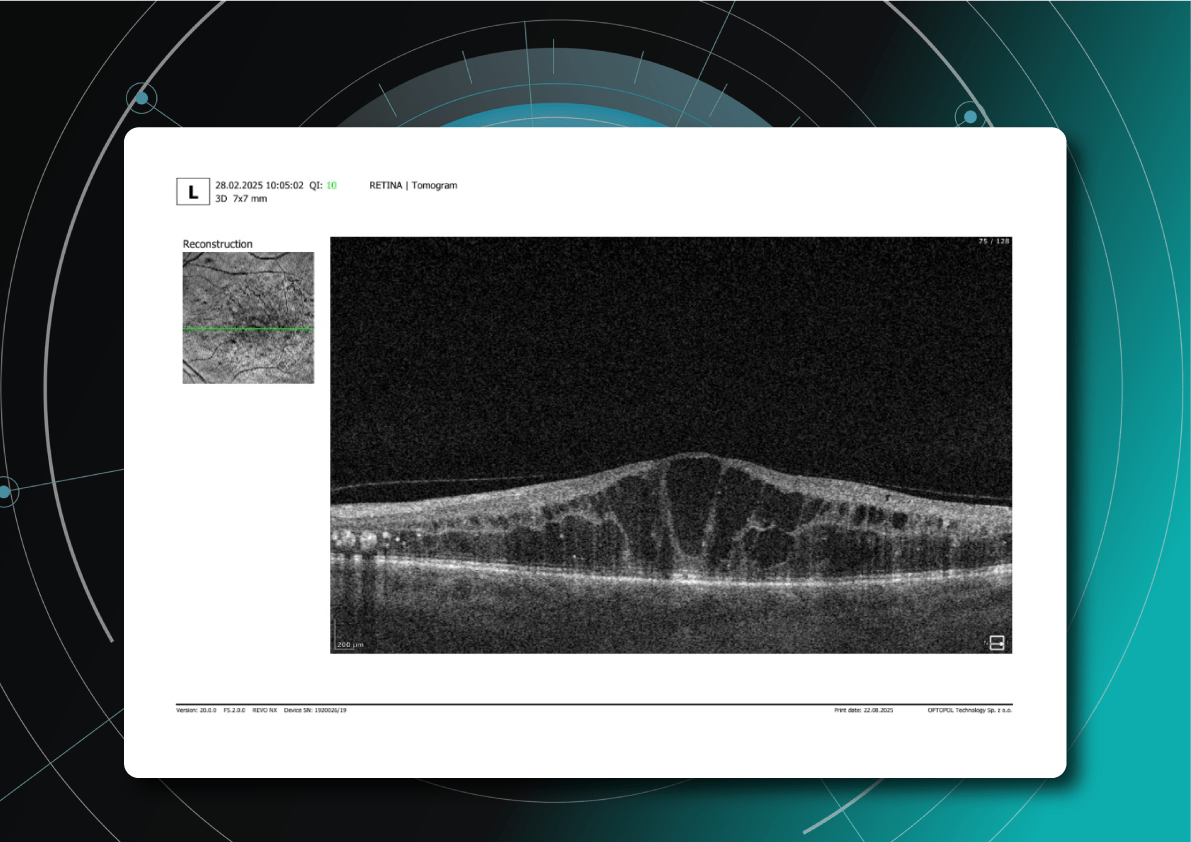
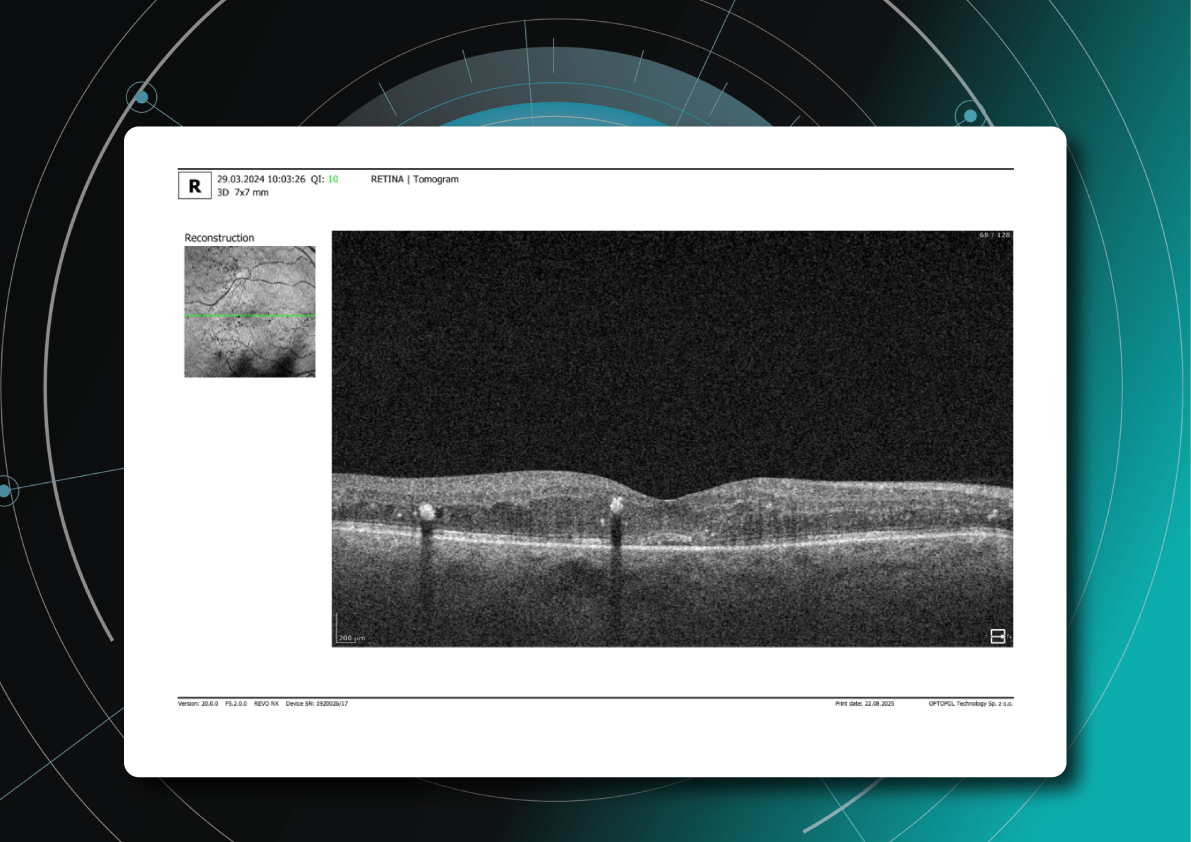
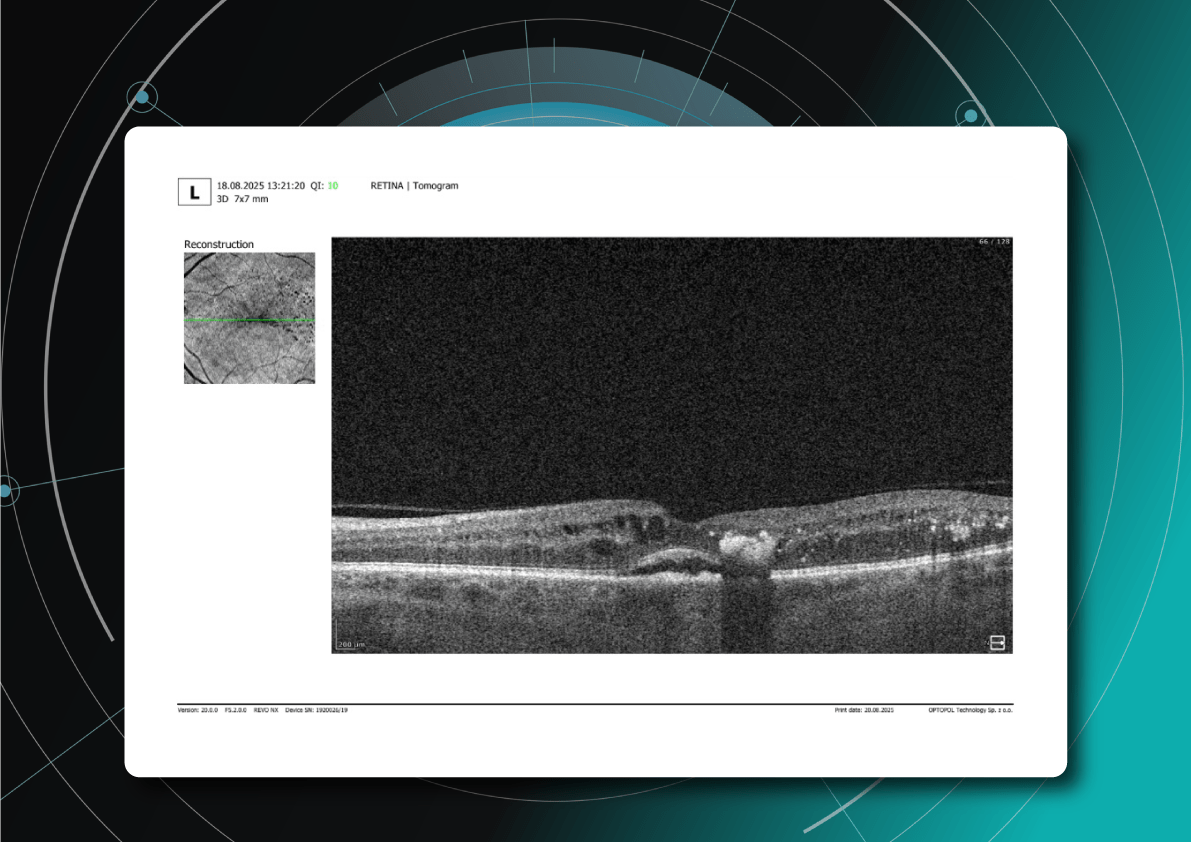






















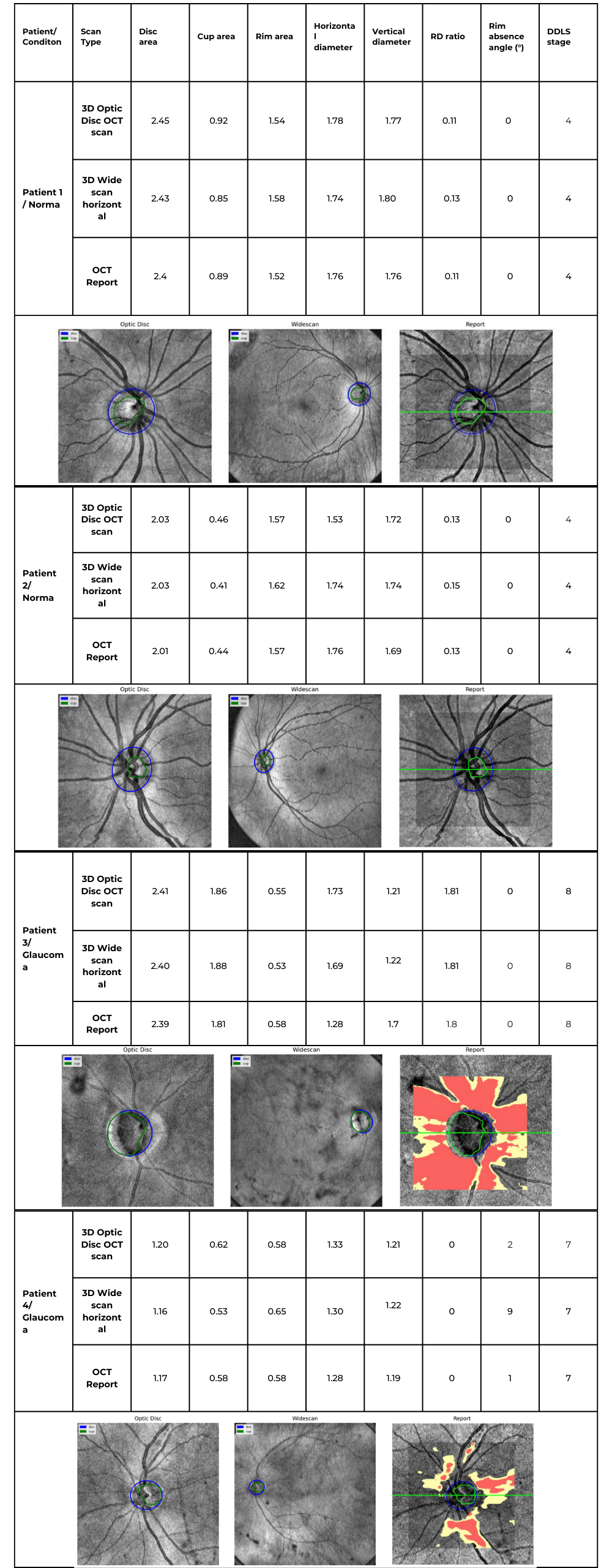
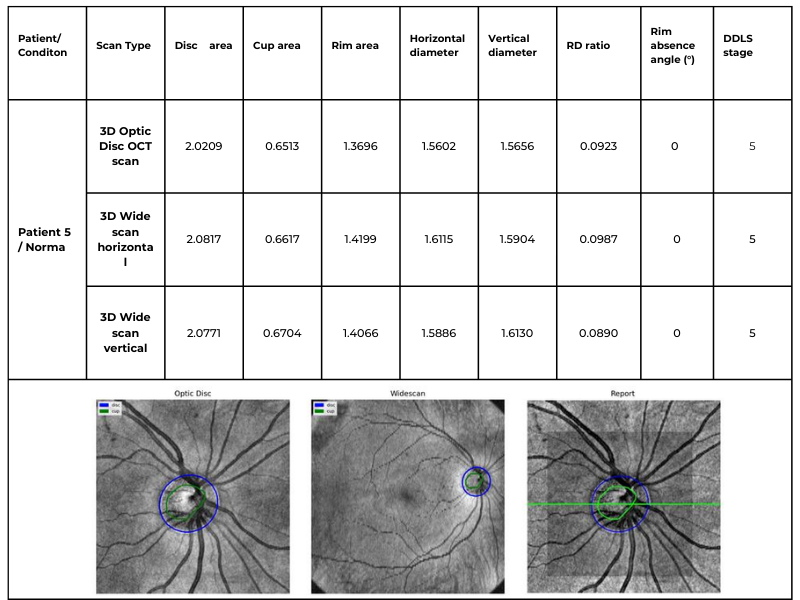
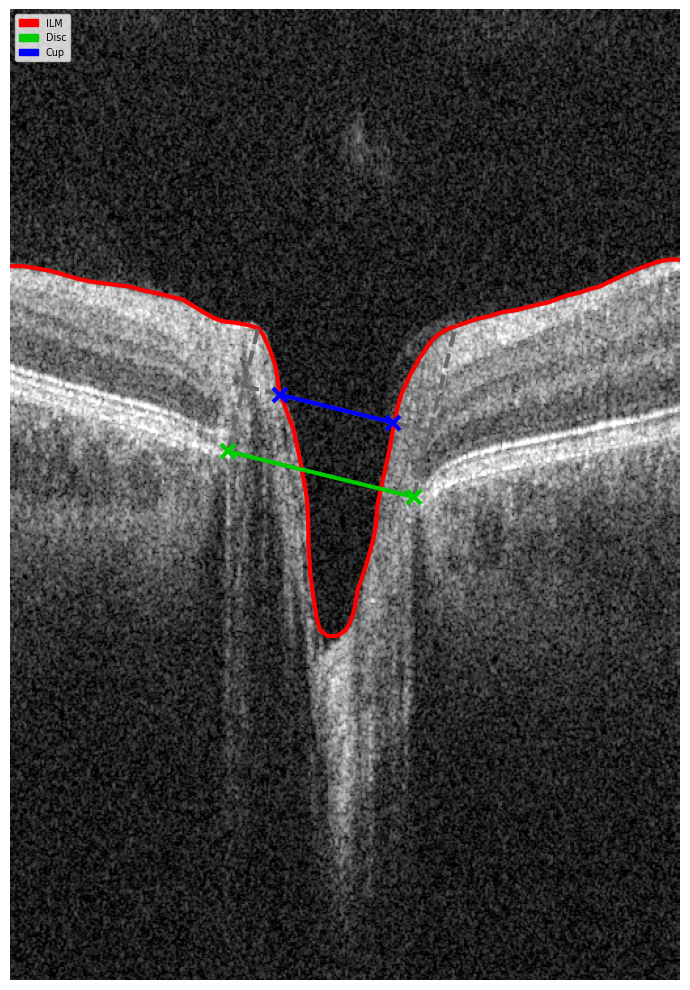































 As David Parkins, the ex-president of the College of Optometrists,
As David Parkins, the ex-president of the College of Optometrists,